Packaging of the virion host shutoff (Vhs) protein of herpes simplex virus: two forms of the Vhs polypeptide are associated with intranuclear B and C capsids, but only one is associated with enveloped virions
- PMID: 17093196
- PMCID: PMC1797492
- DOI: 10.1128/JVI.01812-06
Packaging of the virion host shutoff (Vhs) protein of herpes simplex virus: two forms of the Vhs polypeptide are associated with intranuclear B and C capsids, but only one is associated with enveloped virions
Abstract
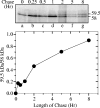
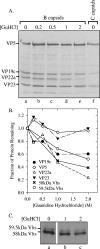
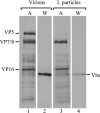
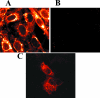
The virion host shutoff (Vhs) protein (UL41) is a minor component of herpes simplex virus virions which, following penetration, accelerates turnover of host and viral mRNAs. Infected cells contain 58-kDa and 59.5-kDa forms of Vhs, which differ in the extent of phosphorylation, yet only a 58-kDa polypeptide is incorporated into virions. In pulse-chase experiments, the primary Vhs translation product comigrated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis with the 58-kDa virion polypeptide, and could be chased to 59.5 kDa. While both 59.5-kDa and 58-kDa forms were found in nuclear and cytoplasmic fractions, the 59.5-kDa form was significantly enriched in the nucleus. Both forms were associated with intranuclear B and C capsids, yet only the 58-kDa polypeptide was found in enveloped cytoplasmic virions. A 58-kDa form, but not the 59.5-kDa form, was found in L particles, noninfectious particles that contain an envelope and tegument but no capsid. The data suggest that virions contain two populations of Vhs that are packaged by different pathways. In the first pathway, the primary translation product is processed to 59.5 kDa, is transported to the nucleus, binds intranuclear capsids, and is converted to 58 kDa at some stage prior to final envelopment. The second pathway does not involve the 59.5-kDa form or interactions between Vhs and capsids. Instead, the primary translation product is phosphorylated to the 58-kDa virion form and packaged through interactions with other tegument proteins in the cytoplasm or viral envelope proteins at the site of final envelopment.
Figures








References
-
- Boyle, W. J., P. Van Der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates, p. 110-149. In T. Hunter and B. M. Sefton (ed.), Protein phosphorylation, part B. Academic Press, Inc., San Diego, CA. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

