Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney
- PMID: 17093497
- PMCID: PMC1679761
- DOI: 10.1038/sj.emboj.7601421
Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney
Abstract
During embryonic development, the kidney epithelium originates from cells that undergo a mesenchymal to epithelial transition (MET). The reverse process, epithelium to mesenchyme transition (EMT), has been implicated in epithelial tumor progression and in the fibrosis that leads to end-stage kidney failure. Snail transcription factors induce both natural and pathological EMT, but their implication in renal development and disease is still unclear. We show that Snail genes are downregulated during the MET that occurs during renal development and that this is correlated with Cadherin-16 expression. Snail suppresses Cadherin-16 via the direct repression of the kidney differentiation factor HNF-1beta, a novel route by which Snail disrupts epithelial homeostasis. Indeed, Snail activation is sufficient to induce EMT and kidney fibrosis in adult transgenic mice. Significantly, Snail is also activated in patients with renal fibrosis. Thus, Snail expression is suppressed during renal development and it must remain silent in the mature kidney where its aberrant activation leads to fibrosis.
Figures

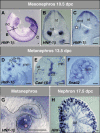



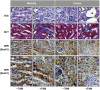

References
-
- Alexakis C, Maxwell P, Bou-Gharios G (2005) Organ-specific collagen expression: implications for renal disease. Nephron Exp Nephrol 102: e71–e75 - PubMed
-
- Aybar M, Nieto MA, Mayor R (2003) Snail precedes Slug in the genetic cascade required for the specification and migration of the Xenopus neural crest. Development 130: 483–494 - PubMed
-
- Bai Y, Pontoglio M, Hiesberger T, Sinclair AM, Igarashi P (2002) Regulation of kidney-specific Ksp-cadherin gene promoter by hepatocyte nuclear factor-1β. Am J Physiol Renal Physiol 283: F839–F851 - PubMed
-
- Barrallo-Gimeno A, Nieto MA (2005) The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132: 3151–3161 - PubMed
-
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A (2000) The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2: 84–89 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

