An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection
- PMID: 17095599
- PMCID: PMC1635544
- DOI: 10.1073/pnas.0608578103
An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection
Abstract
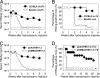
An animal model for human hepatitis B virus (HBV) tolerance is needed to investigate the mechanisms. This model will also facilitate therapeutic strategies for the existing 350 million patients with chronic hepatitis B. We established a mouse model by hydrodynamic injection of an engineered, replication-competent HBV DNA into the tail veins of C57BL/6 mice. In 40% of the injected mice, HBV surface antigenemia persisted for > 6 months. Viral replication intermediates, transcripts, and proteins were detected in the liver tissues of the injected mice for up to 1 year. The tolerance toward HBV surface antigen in this model was shown to be due to an insufficient cellular immunity against hepatitis B core antigen, as was documented in humans. This animal model will accelerate further genetic and mechanistic studies of human chronic hepatitis B infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


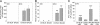

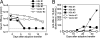
References
-
- Chisari FV, Ferrari C. Annu Rev Immunol. 1995;13:29–60. - PubMed
-
- Moriyama T, Guilhot S, Klopchin K, Moss B, Pinkert CA, Palmiter RD, Brinster RL, Kanagawa O, Chisari FV. Science. 1990;248:361–364. - PubMed
-
- Larkin J, Clayton M, Sun B, Perchonock CE, Morgan JL, Siracusa LD, Michaels FH, Feitelson MA. Nat Med. 1999;5:907–912. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

