Src family kinases mediate neutrophil adhesion to adherent platelets
- PMID: 17095622
- PMCID: PMC1852189
- DOI: 10.1182/blood-2006-06-029082
Src family kinases mediate neutrophil adhesion to adherent platelets
Abstract
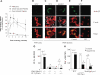
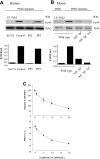
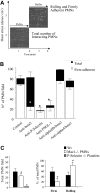
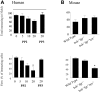
Polymorphonuclear leukocyte (PMN)-platelet interactions at sites of vascular damage contribute to local and systemic inflammation. We sought to determine the role of "outside-in" signaling by Src-family tyrosine kinases (SFKs) in the regulation of alphaMbeta2-integrin-dependent PMN recruitment by activated platelets under (patho)physiologic conditions. Activation-dependent epitopes in beta2 integrin were exposed at the contact sites between PMNs and platelets and were abolished by SFK inhibitors. PMNs from alphaMbeta2(-/-), hck(-/-)fgr(-/-), and hck(-/-)fgr(-/-)lyn(-/-) mice had an impaired capacity to adhere with activated platelets in suspension. Phosphorylation of Pyk2 accompanied PMN adhesion to platelets and was blocked by inhibition as well as by genetic deletion of alphaMbeta2 integrin and SFKs. A Pyk2 inhibitor reduced platelet-PMN adhesion, indicating that Pyk2 may be a downstream effector of SFKs. Analysis of PMN-platelet interactions under flow revealed that SFK signaling was required for alphaMbeta2-mediated shear-resistant adhesion of PMNs to adherent platelets, but was dispensable for P-selectin-PSGL-1-mediated recruitment and rolling. Finally, SFK activity was required to support PMN accumulation along adherent platelets at the site of vascular injury, in vivo. These results definitely establish a role for SFKs in PMN recruitment by activated platelets and suggest novel targets to disrupt the pathophysiologic consequences of platelet-leukocyte interactions in vascular disease.
Figures


References
-
- Weyrich AS, Prescott SM, Zimmerman GA. Platelets, endothelial cells, inflammatory chemokines, and restenosis: complex signaling in the vascular play book. Circulation. 2002;106:1433–1435. - PubMed
-
- Theilmeier G, Michiels C, Spaepen E, et al. Endothelial von Willebrand factor recruits platelets to atherosclerosis-prone sites in response to hypercholesterolemia. Blood. 2002;99:4486–4493. - PubMed
-
- Huo Y, Schober A, Forlow SB, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. - PubMed
-
- Smyth SS, Reis ED, Zhang W, Fallon JT, Gordon RE, Coller BS. b3-integrin-deficient mice but not P-selectin-deficient mice develop intimal hyperplasia after vascular injury: correlation with leukocyte recruitment to adherent platelets 1 hour after injury. Circulation. 2001;103:2501–2507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

