Oral premalignant lesions induce immune reactivity to both premalignant oral lesions and head and neck squamous cell carcinoma
- PMID: 17096152
- PMCID: PMC11029909
- DOI: 10.1007/s00262-006-0242-7
Oral premalignant lesions induce immune reactivity to both premalignant oral lesions and head and neck squamous cell carcinoma
Abstract
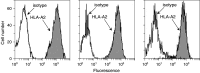
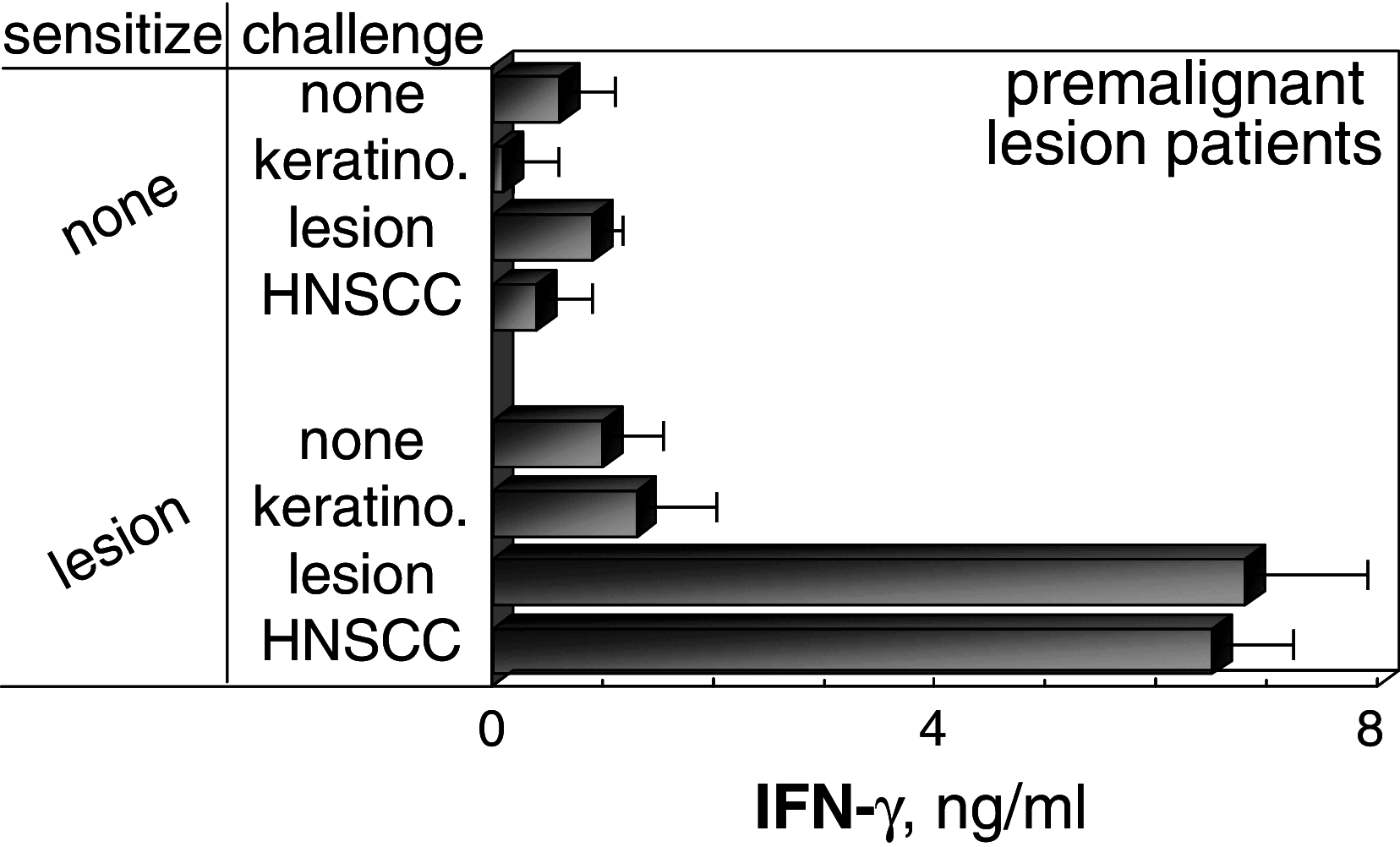
Head and neck squamous cell carcinoma (HNSCC) is an aggressive malignancy, and despite advances in treatments, the 5-year survival has remained at less than 50%. One treatment strategy is to focus on patients with premalignant oral lesions that carry a high-risk for developing recurrent premalignant lesions and HNSCC disease. As an initial attempt to determine if immune therapy has the potential to be protective in these patients, studies determined if premalignant lesions express tumor antigens that have previously been shown to be expressed on HNSCC. Immunohistochemical analyses showed prominent expression of epidermal growth factor receptor in premalignant lesions, even in lesions with mild dysplasia. MUC-1 and carcinoembryonic antigen were expressed in most patient samples, while NY-ESO-1 was less frequently expressed. Each of these antigens was expressed on HNSCC. This provided the rationale for determining if premalignant oral lesions could be used to stimulate autologous peripheral blood mononuclear leukocytes (PBML) to react against heterologous premalignant lesions and HNSCC. Following sensitization with autologous premalignant lesions, PBML responded to a challenge with either heterologous premalignant oral lesion cells or HNSCC by releasing IFN-gamma. In addition, sensitization with autologous premalignant lesion lysates generated cytolytic activity by both PBML and T cells against allogeneic premalignant lesion cells and HNSCC. These studies show the feasibility of using premalignant oral lesions to stimulate immune reactivity against both premalignant oral lesions as well as HNSCC.
Figures






References
-
- Araujo CS, Graner E, Almeida OP, Sauk JJ, Coletta RD. Histomorphometric characteristics and expression of epidermal growth factor and its receptor by epithelial cells of normal gingiva and hereditary gingival fibromatosis. J Periodontal Res. 2003;38:237–241. doi: 10.1034/j.1600-0765.2003.00013.x. - DOI - PubMed
-
- Bouquot JE, Whitaker SB. Oral leukoplakia—rationale for diagnosis and prognosis of its clinical subtypes or “phases”. Quintessence Int. 1994;25:133–140. - PubMed
-
- Bruzzese F, Di Gennaro E, Avallone A, Pepe S, Arra C, Caraglia M, Tagliaferri P, Budillon A. Synergistic antitumor activity of epidermal growth factor receptor tyrosine kinase inhibitor gefitinib and IFN-α in head and neck cancer cells in vitro and in vivo. Clin Cancer Res. 2006;12:617–625. doi: 10.1158/1078-0432.CCR-05-1671. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

