A common signaling cascade may underlie "addiction" to the Src, BCR-ABL, and EGF receptor oncogenes
- PMID: 17097564
- PMCID: PMC2673136
- DOI: 10.1016/j.ccr.2006.09.014
A common signaling cascade may underlie "addiction" to the Src, BCR-ABL, and EGF receptor oncogenes
Erratum in
- Cancer Cell. 2009 Nov 6;16(5):448
Abstract
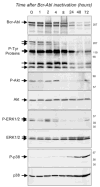
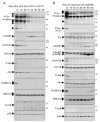
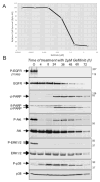
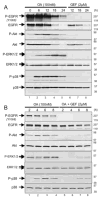
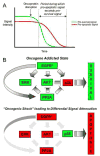
"Oncogene addiction" describes an unexplained dependency of cancer cells on a particular cellular pathway for survival or proliferation. We report that differential attenuation rates of prosurvival and proapoptotic signals in oncogene-dependent cells contribute to cell death following oncogene inactivation. Src-, BCR-ABL-, and EGF receptor-dependent cells exhibit a similar profile of signal attenuation following oncogene inactivation characterized by rapid diminution of phospho-ERK, -Akt, and -STAT3/5, and a delayed accumulation of the proapoptotic effector phospho-p38 MAPK. These findings implicate a transient imbalance in survival and apoptotic oncogenic outputs in the apoptotic response to oncogene inactivation. Moreover, these observations implicate a common profile of signal attenuation for multiple oncogenes and suggest that "addiction" associated with apoptosis reflects an active rather than a passive process.
Figures



Comment in
-
Can't kick that oncogene habit.Cancer Cell. 2006 Nov;10(5):345-7. doi: 10.1016/j.ccr.2006.10.013. Cancer Cell. 2006. PMID: 17097554
Similar articles
-
Systems biology modeling reveals a possible mechanism of the tumor cell death upon oncogene inactivation in EGFR addicted cancers.PLoS One. 2011;6(12):e28930. doi: 10.1371/journal.pone.0028930. Epub 2011 Dec 14. PLoS One. 2011. PMID: 22194952 Free PMC article.
-
Selective pyrrolo-pyrimidine inhibitors reveal a necessary role for Src family kinases in Bcr-Abl signal transduction and oncogenesis.Oncogene. 2002 Nov 21;21(53):8075-88. doi: 10.1038/sj.onc.1206008. Oncogene. 2002. PMID: 12444544
-
"Oncogenic shock": explaining oncogene addiction through differential signal attenuation.Clin Cancer Res. 2006 Jul 15;12(14 Pt 2):4392s-4395s. doi: 10.1158/1078-0432.CCR-06-0096. Clin Cancer Res. 2006. PMID: 16857816 Review.
-
Mammary gland specific expression of Brk/PTK6 promotes delayed involution and tumor formation associated with activation of p38 MAPK.Breast Cancer Res. 2011 Sep 17;13(5):R89. doi: 10.1186/bcr2946. Breast Cancer Res. 2011. PMID: 21923922 Free PMC article.
-
Last findings on dual inhibitors of abl and SRC tyrosine-kinases.Mini Rev Med Chem. 2007 Feb;7(2):191-201. doi: 10.2174/138955707779802598. Mini Rev Med Chem. 2007. PMID: 17305593 Review.
Cited by
-
Alterations of Gab2 signalling complexes in imatinib and dasatinib treated chronic myeloid leukaemia cells.Cell Commun Signal. 2013 Apr 22;11(1):30. doi: 10.1186/1478-811X-11-30. Cell Commun Signal. 2013. PMID: 23607741 Free PMC article.
-
The STAT5 Inhibitor Pimozide Displays Efficacy in Models of Acute Myelogenous Leukemia Driven by FLT3 Mutations.Genes Cancer. 2012 Jul;3(7-8):503-11. doi: 10.1177/1947601912466555. Genes Cancer. 2012. PMID: 23264850 Free PMC article.
-
Clinical implications of novel activating EGFR mutations in malignant peritoneal mesothelioma.World J Surg Oncol. 2010 Oct 13;8:88. doi: 10.1186/1477-7819-8-88. World J Surg Oncol. 2010. PMID: 20942962 Free PMC article.
-
Targeting TAZ-Driven Human Breast Cancer by Inhibiting a SKP2-p27 Signaling Axis.Mol Cancer Res. 2019 Jan;17(1):250-262. doi: 10.1158/1541-7786.MCR-18-0332. Epub 2018 Sep 20. Mol Cancer Res. 2019. PMID: 30237296 Free PMC article.
-
Perturbation of the mutated EGFR interactome identifies vulnerabilities and resistance mechanisms.Mol Syst Biol. 2013 Nov 5;9:705. doi: 10.1038/msb.2013.61. Mol Syst Biol. 2013. PMID: 24189400 Free PMC article.
References
-
- Aoki K, Yoshida T, Matsumoto N, Ide H, Sugimura T, Terada M. Suppression of Ki-ras p21 levels leading to growth inhibition of pancreatic cancer cell lines with Ki-ras mutation but not those without Ki-ras mutation. Mol Carcinog. 1997;20:251–258. - PubMed
-
- Arber N. Janus faces of ras: anti or pro-apoptotic? Apoptosis. 1999;4:383–388. - PubMed
-
- Bandyopadhyay G, Biswas T, Roy KC, Mandal S, Mandal C, Pal BC, Bhattacharya S, Rakshit S, Bhattacharya DK, Chaudhuri U, et al. Chlorogenic acid inhibits Bcr-Abl tyrosine kinase and triggers p38 mitogen-activated protein kinase-dependent apoptosis in chronic myelogenous leukemic cells. Blood. 2004;104:2514–2522. - PubMed
-
- Brunet CL, Gunby RH, Benson RS, Hickman JA, Watson AJ, Brady G. Commitment to cell death measured by loss of clonogenicity is separable from the appearance of apoptotic markers. Cell Death Differ. 1998;5:107–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

