Promotion of tubulin assembly by poorly soluble taxol analogs
- PMID: 17097592
- PMCID: PMC1868410
- DOI: 10.1016/j.ab.2006.10.014
Promotion of tubulin assembly by poorly soluble taxol analogs
Abstract
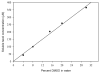
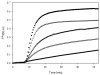
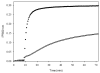
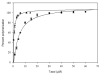
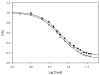
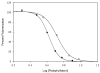
Promotion or inhibition of tubulin assembly into microtubules is the standard in vitro assay for evaluating potential antimicrotubule agents. Many agents to be tested are poorly soluble in aqueous solution and require a cosolvent such as dimethyl sulfoxide (DMSO). However, DMSO itself can promote tubulin assembly, and its inclusion in assays for compounds that induce tubulin assembly complicates interpretation of the results. Substituting GDP for GTP in the exchangeable nucleotide binding site of tubulin produces a less active form of the protein, tubulin-GDP. Here it is shown that tubulin-GDP can be assembled into normal microtubules in DMSO concentrations up to 15% (v/v), and polymerization assays performed under these conditions can be compared with assays run under more standard conditions. Assays for measuring the effective concentration of a ligand for promotion of tubulin assembly (EC(50)), measuring the concentration for inhibition of tubulin assembly (IC(50)) by a colchicine site ligand, and measuring tubulin critical concentrations in the presence of poorly soluble taxol derivatives are illustrated.
Figures
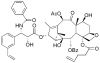

References
-
- Bhalla KN. Microtubule – targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–9086. - PubMed
-
- Wilson L, Jordan MA. New microtubule/tubulin-targeted anticancer drugs and novel chemotherapeutic strategies. J Chemother. 2004;4(16 Suppl):83–85. - PubMed
-
- Zhou J, Giannakakou P. Targeting microtubules for cancer chemotherapy. Curr Med Chem: Anti-Cancer Agents. 2005;5:65–71. - PubMed
-
- Diaz JF, Andreu JM. Assembly of purified GDP-tubulin into microtubules induced by taxol and taxotere: reversibility, ligand stoichiometry, and competition. Biochemistry. 1993;32:2747–2755. - PubMed
-
- Verdier-Pinard P, Wang Z, Mohanakrishnan AK, Cushman M, Hamel E. A steroid derivative with paclitaxel-like effects on tubulin polymerization. Mol Pharm. 2000;57:568–575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

