Parkin is an E3 ubiquitin-ligase for normal and mutant ataxin-2 and prevents ataxin-2-induced cell death
- PMID: 17097639
- PMCID: PMC2788988
- DOI: 10.1016/j.expneurol.2006.09.009
Parkin is an E3 ubiquitin-ligase for normal and mutant ataxin-2 and prevents ataxin-2-induced cell death
Abstract
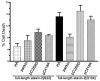
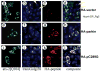
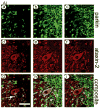
Expansion of the polyQ repeat in ataxin-2 results in degeneration of Purkinje neurons and other neuronal groups including the substantia nigra in patients with spinocerebellar ataxia type 2 (SCA2). In animal and cell models, overexpression of mutant ataxin-2 induces cell dysfunction and death, but little is known about steady-state levels of normal and mutant ataxin-2 and cellular mechanisms regulating their abundance. Based on preliminary findings that ataxin-2 interacted with parkin, an E3 ubiquitin ligase mutated in an autosomal recessive form of Parkinsonism, we sought to determine whether parkin played a role in regulating the steady-state levels of ataxin-2. Parkin interacted with the N-terminal half of normal and mutant ataxin-2, and ubiquitinated the full-length form of both wild-type and mutant ataxin-2. Parkin also regulated the steady-state levels of endogenous ataxin-2 in PC12 cells with regulatable parkin expression. Parkin reduced abnormalities in Golgi morphology induced by mutant ataxin-2 and decreased ataxin-2 induced cytotoxicity. In brains of SCA2 patients, parkin labeled cytoplasmic ataxin-2 aggregates in Purkinje neurons. These studies suggest a role for parkin in regulating the intracellular levels of both wild-type and mutant ataxin-2, and in rescuing cells from ataxin-2-induced cytotoxicity. The role of parkin variants in modifying the SCA2 phenotype and its use as a therapeutic target should be further investigated.
Figures






Similar articles
-
Expansion of the polyQ repeat in ataxin-2 alters its Golgi localization, disrupts the Golgi complex and causes cell death.Hum Mol Genet. 2003 Jul 1;12(13):1485-96. doi: 10.1093/hmg/ddg175. Hum Mol Genet. 2003. PMID: 12812977
-
Ataxin-2 mediated cell death is dependent on domains downstream of the polyQ repeat.Exp Neurol. 2007 Dec;208(2):207-15. doi: 10.1016/j.expneurol.2007.07.023. Epub 2007 Aug 28. Exp Neurol. 2007. PMID: 17949716 Free PMC article.
-
Progress in pathogenesis studies of spinocerebellar ataxia type 1.Philos Trans R Soc Lond B Biol Sci. 1999 Jun 29;354(1386):1079-81. doi: 10.1098/rstb.1999.0462. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10434309 Free PMC article. Review.
-
Mutation of the E6-AP ubiquitin ligase reduces nuclear inclusion frequency while accelerating polyglutamine-induced pathology in SCA1 mice.Neuron. 1999 Dec;24(4):879-92. doi: 10.1016/s0896-6273(00)81035-1. Neuron. 1999. PMID: 10624951
-
Role of inositol 1,4,5-trisphosphate receptors in pathogenesis of Huntington's disease and spinocerebellar ataxias.Neurochem Res. 2011 Jul;36(7):1186-97. doi: 10.1007/s11064-010-0393-y. Epub 2011 Jan 6. Neurochem Res. 2011. PMID: 21210219 Free PMC article. Review.
Cited by
-
Spinocerebellar ataxia 2 (SCA2).Cerebellum. 2008;7(2):115-24. doi: 10.1007/s12311-008-0019-y. Cerebellum. 2008. PMID: 18418684 Review.
-
From pathways to targets: understanding the mechanisms behind polyglutamine disease.Biomed Res Int. 2014;2014:701758. doi: 10.1155/2014/701758. Epub 2014 Sep 21. Biomed Res Int. 2014. PMID: 25309920 Free PMC article. Review.
-
Loss of parkin reduces lung tumor development by blocking p21 degradation.PLoS One. 2019 May 21;14(5):e0217037. doi: 10.1371/journal.pone.0217037. eCollection 2019. PLoS One. 2019. PMID: 31112565 Free PMC article.
-
The Role of Protein Quantity Control in Polyglutamine Spinocerebellar Ataxias.Cerebellum. 2024 Dec;23(6):2575-2592. doi: 10.1007/s12311-024-01722-w. Epub 2024 Jul 25. Cerebellum. 2024. PMID: 39052145 Review.
-
A Novel Duplication in ATXN2 as Modifier for Spinocerebellar Ataxia 3 (SCA3) and C9ORF72-ALS.Mov Disord. 2021 Feb;36(2):508-514. doi: 10.1002/mds.28334. Epub 2020 Oct 15. Mov Disord. 2021. PMID: 33058338 Free PMC article.
References
-
- Abbas N, Lucking CB, Ricard S, Durr A, Bonifati V, De Michele G, Bouley S, Vaughan JR, Gasser T, Marconi R, Broussolle E, Brefel-Courbon C, Harhangi BS, Oostra BA, Fabrizio E, Bohme GA, Pradier L, Wood NW, Filla A, Meco G, Denefle P, Agid Y, Brice A. A wide variety of mutations in the parkin gene are responsible for autosomal recessive parkinsonism in Europe. French Parkinson's Disease Genetics Study Group and the European Consortium on Genetic Susceptibility in Parkinson's Disease. Hum Mol Genet. 1999;8:567–574. - PubMed
-
- Armstrong J, Bonaventura I, Rojo A, Gonzalez G, Corral J, Nadal N, Volpini V, Ferrer I. Spinocerebellar ataxia type 2 (SCA2) with white matter involvement. Neurosci Lett. 2005;381:247–251. - PubMed
-
- Bingol B, Schuman EM. Activity-dependent dynamics and sequestration of proteasomes in dendritic spines. Nature. 2006;441:1144–1148. - PubMed
-
- Chung KK, Zhang Y, Lim KL, Tanaka Y, Huang H, Gao J, Ross CA, Dawson VL, Dawson TM. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med. 2001;7:1144–1150. - PubMed
-
- Corti O, Hampe C, Koutnikova H, Darios F, Jacquier S, Prigent A, Robinson JC, Pradier L, Ruberg M, Mirande M, Hirsch E, Rooney T, Fournier A, Brice A. The p38 subunit of the aminoacyl-tRNA synthetase complex is a Parkin substrate: linking protein biosynthesis and neurodegeneration. Hum Mol Genet. 2003;12:1427–1437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

