Characterization of hemocytes from the mosquitoes Anopheles gambiae and Aedes aegypti
- PMID: 17098164
- PMCID: PMC2757042
- DOI: 10.1016/j.ibmb.2006.08.010
Characterization of hemocytes from the mosquitoes Anopheles gambiae and Aedes aegypti
Abstract
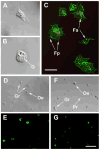
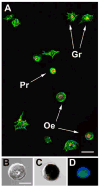
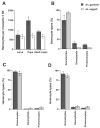
Hemocytes are an essential component of the mosquito immune system but current knowledge of the types of hemocytes mosquitoes produce, their relative abundance, and their functions is limited. Addressing these issues requires improved methods for collecting and maintaining mosquito hemocytes in vitro, and comparative data that address whether important vector species produce similar or different hemocyte types. Toward this end, we conducted a comparative study with Anopheles gambiae and Aedes aegypti. Collection method greatly affected the number of hemocytes and contaminants obtained from adult females of each species. Using a collection method called high injection/recovery, we concluded that hemolymph from An. gambiae and Ae. aegypti adult females contains three hemocyte types (granulocytes, oenocytoids and prohemocytes) that were distinguished from one another by a combination of morphological and functional markers. Significantly more hemocytes were recovered from An. gambiae females than Ae. aegypti. However, granulocytes were the most abundant cell type in both species while oenocytoids and prohemocytes comprised less than 10% of the total hemocyte population. The same hemocyte types were collected from larvae, pupae and adult males albeit the absolute number and proportion of each hemocyte type differed from adult females. The number of hemocytes recovered from sugar fed females declined with age but blood feeding transiently increased hemocyte abundance. Two antibodies tested as potential hemocyte markers (anti-PP06 and anti-Dox-A2) also exhibited alterations in staining patterns following immune challenge with the bacterium Escherichia coli.
Figures




References
-
- Andradis TG, Hall DW. Neoplectana carpocapsae: encapsulation in Aedes aegypti and changes in host hemocytes and hemolymph proteins. Experimental Parasitology. 1976;39:252–261. - PubMed
-
- Ashida M, Ochiae M, Niki T. Immunolocalization of prophenoloxidase among hemocytes of the silkworm Bombyx mori. Tissue and Cell. 1988;20:599–610. - PubMed
-
- Bartholomay LC, Cho WL, Rocheleau TA, Boyle JP, Beck ET, Fuchs JF, Liss P, Rusch M, Butler KM, Wu RCC, Lin SP, Kuo HY, Tsao IY, Huang CY, Liu TT, Hsiao KJ, Tsai SF, Yang UC, Nappi AJ, Perna NT, Chen CC, Christensen BM. Description of the transcriptomes of immune response-activated hemocytes from the mosquito vectors Aedes aegypti and Armigeres subalbatus. Infection and Immunity. 2004;72:4114–4126. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

