Arabidopsis SAMT1 defines a plastid transporter regulating plastid biogenesis and plant development
- PMID: 17098813
- PMCID: PMC1693945
- DOI: 10.1105/tpc.105.040741
Arabidopsis SAMT1 defines a plastid transporter regulating plastid biogenesis and plant development
Abstract
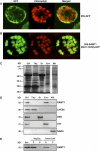
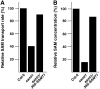
S-Adenosylmethionine (SAM) is formed exclusively in the cytosol but plays a major role in plastids; SAM can either act as a methyl donor for the biogenesis of small molecules such as prenyllipids and macromolecules or as a regulator of the synthesis of aspartate-derived amino acids. Because the biosynthesis of SAM is restricted to the cytosol, plastids require a SAM importer. However, this transporter has not yet been identified. Here, we report the molecular and functional characterization of an Arabidopsis thaliana gene designated SAM TRANSPORTER1 (SAMT1), which encodes a plastid metabolite transporter required for the import of SAM from the cytosol. Recombinant SAMT1 produced in yeast cells, when reconstituted into liposomes, mediated the counter-exchange of SAM with SAM and with S-adenosylhomocysteine, the by-product and inhibitor of transmethylation reactions using SAM. Insertional mutation in SAMT1 and virus-induced gene silencing of SAMT1 in Nicotiana benthamiana caused severe growth retardation in mutant plants. Impaired function of SAMT1 led to decreased accumulation of prenyllipids and mainly affected the chlorophyll pathway. Biochemical analysis suggests that the latter effect represents one prominent example of the multiple events triggered by undermethylation, when there is decreased SAM flux into plastids.
Figures



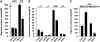


References
-
- Aarnes, H. (1978). Regulation of threonine biosynthesis in barley seedlings (Hordeum vulgare L.). Planta 140 185–192. - PubMed
-
- Agrimi, G., Di Noia, M.A., Marobbio, C.M., Fiermonte, G., Lasorsa, F.M., and Palmieri, F. (2004). Identification of the human mitochondrial S-adenosylmethionine transporter: Bacterial expression, reconstitution, functional characterization and tissue distribution. Biochem. J. 379 183–190. - PMC - PubMed
-
- Alawady, A.E., and Grimm, B. (2005). Tobacco Mg protoporphyrin IX methyltransferase is involved in inverse activation of Mg porphyrin and protoheme synthesis. Plant J. 41 282–290. - PubMed
-
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

