The influence of previous exposure to environmental mycobacteria on the interferon-gamma response to bacille Calmette-Guérin vaccination in southern England and northern Malawi
- PMID: 17100757
- PMCID: PMC1810413
- DOI: 10.1111/j.1365-2249.2006.03222.x
The influence of previous exposure to environmental mycobacteria on the interferon-gamma response to bacille Calmette-Guérin vaccination in southern England and northern Malawi
Abstract
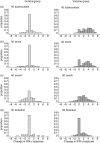
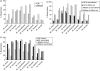
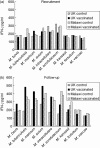
We report a large study of the effect of BCG vaccination on the in vitro 6-day whole blood interferon-gamma (IFN-gamma) response to antigens from eight species of mycobacteria among schoolchildren in south-eastern England, where bacille Calmette-Guérin (BCG) vaccination is highly protective against pulmonary tuberculosis, and among young adults in northern Malawi, where BCG vaccination is not protective. In the UK children, BCG induced an appreciable increase in IFN-gamma response to antigens from most species of mycobacteria. The degree of change was linked to the relatedness of the species to Mycobacterium bovis BCG, and provides further evidence of the cross-reactivity of mycobacterial species in priming of the immune system. IFN-gamma responses to purified protein derivatives (PPDs) from M. tuberculosis and environmental mycobacteria were more prevalent in the Malawian than the UK group prior to vaccination; BCG vaccination increased the prevalence of responses to these PPDs in the UK group to a level similar to that in Malawi. There was no evidence that the vaccine-induced change in IFN-gamma response was dependent upon the magnitude of the initial response of the individual to environmental mycobacteria in the United Kingdom or in Malawi. These observations should assist the development and interpretation of human clinical trials of new vaccines against M. tuberculosis in areas of both low and high exposure to environmental mycobacteria.
Figures

Similar articles
-
Patterns and implications of naturally acquired immune responses to environmental and tuberculous mycobacterial antigens in northern Malawi.J Infect Dis. 2001 Aug 1;184(3):322-9. doi: 10.1086/322042. Epub 2001 Jul 10. J Infect Dis. 2001. PMID: 11443558 Clinical Trial.
-
Gamma interferon responses induced by a panel of recombinant and purified mycobacterial antigens in healthy, non-mycobacterium bovis BCG-vaccinated Malawian young adults.Clin Diagn Lab Immunol. 2003 Jul;10(4):602-11. doi: 10.1128/cdli.10.4.602-611.2003. Clin Diagn Lab Immunol. 2003. PMID: 12853392 Free PMC article.
-
BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies.Lancet. 2002 Apr 20;359(9315):1393-401. doi: 10.1016/S0140-6736(02)08353-8. Lancet. 2002. PMID: 11978337 Clinical Trial.
-
Tuberculosis vaccines: beyond bacille Calmette-Guerin.Philos Trans R Soc Lond B Biol Sci. 2011 Oct 12;366(1579):2782-9. doi: 10.1098/rstb.2011.0097. Philos Trans R Soc Lond B Biol Sci. 2011. PMID: 21893541 Free PMC article. Review.
-
In vitro cellular and cytokine responses to mycobacterial antigens: application to diagnosis of tuberculosis infection and assessment of response to mycobacterial vaccines.Am J Med Sci. 1997 Jun;313(6):364-71. doi: 10.1097/00000441-199706000-00009. Am J Med Sci. 1997. PMID: 9186152 Review.
Cited by
-
Nontuberculous Mycobacteria and Heterologous Immunity to Tuberculosis.J Infect Dis. 2019 Aug 30;220(7):1091-1098. doi: 10.1093/infdis/jiz285. J Infect Dis. 2019. PMID: 31165861 Free PMC article. Review.
-
Increasing the Vaccine Potential of Live M. bovis BCG by Coadministration with Plasmid DNA Encoding a Tuberculosis Prototype Antigen.Vaccines (Basel). 2014 Mar 5;2(1):181-95. doi: 10.3390/vaccines2010181. Vaccines (Basel). 2014. PMID: 26344474 Free PMC article.
-
Oral Tolerance to Environmental Mycobacteria Interferes with Intradermal, but Not Pulmonary, Immunization against Tuberculosis.PLoS Pathog. 2016 May 6;12(5):e1005614. doi: 10.1371/journal.ppat.1005614. eCollection 2016 May. PLoS Pathog. 2016. PMID: 27153120 Free PMC article.
-
Using Data from Macaques To Predict Gamma Interferon Responses after Mycobacterium bovis BCG Vaccination in Humans: a Proof-of-Concept Study of Immunostimulation/Immunodynamic Modeling Methods.Clin Vaccine Immunol. 2017 Mar 6;24(3):e00525-16. doi: 10.1128/CVI.00525-16. Print 2017 Mar. Clin Vaccine Immunol. 2017. PMID: 28077441 Free PMC article.
-
Non-tuberculous mycobacteria immunopathogenesis: Closer than they appear. a prime of innate immunity trade-off and NTM ways into virulence.Scand J Immunol. 2021 Aug;94(2):e13035. doi: 10.1111/sji.13035. Epub 2021 Jun 22. Scand J Immunol. 2021. PMID: 33655533 Free PMC article. Review.
References
-
- Andersen P, Doherty TM. The success and failure of BCG − implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3:656–62. - PubMed
-
- Buddle BM, Wards BJ, Aldwell FE, Collins DM, de Lisle GW. Influence of sensitization to environmental mycobacteria on subsequent vaccination against bovine tuberculosis. Vaccine. 2002;20:1126–33. - PubMed
-
- Edwards ML, Goodrich JM, Muller D, Pollack A, Ziegler JE, Smith DW. Infection with Mycobacterium avium-intracellulare and the protective effects of Bacille Calmette–Guerin. J Infect Dis. 1982;145:733–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

