Vaccination of Alzheimer's model mice with Abeta derivative in alum adjuvant reduces Abeta burden without microhemorrhages
- PMID: 17100841
- PMCID: PMC1779823
- DOI: 10.1111/j.1460-9568.2006.05149.x
Vaccination of Alzheimer's model mice with Abeta derivative in alum adjuvant reduces Abeta burden without microhemorrhages
Abstract
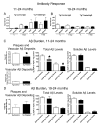
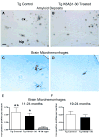
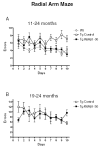
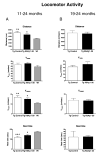
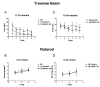
Immunotherapy holds great promise for Alzheimer's disease (AD) and other conformational disorders but certain adverse reactions need to be overcome. The meningoencephalitis observed in the first AD vaccination trial was likely related to excessive cell-mediated immunity caused by the immunogen, amyloid-beta (Abeta) 1-42, and the adjuvant, QS-21. To avoid this toxicity, we have been using Abeta derivatives in alum adjuvant that promotes humoral immunity. Other potential side effects of immunotherapy are increased vascular amyloid and associated microhemorrhages that may be related to rapid clearance of parenchymal amyloid. Here, we determined if our immunization strategy was associated with this form of toxicity, and if the therapeutic effect was age-dependent. Tg2576 mice and wild-type littermates were immunized from 11 or 19 months and their behaviour evaluated prior to killing at 24 months. Subsequently, plaque- and vascular-Abeta burden, Abeta levels and associated pathology was assessed. The therapy started at the cusp of amyloidosis reduced cortical Abeta deposit burden by 31% and Abeta levels by 30-37%, which was associated with cognitive improvements. In contrast, treatment from 19 months, when pathology is well established, was not immunogenic and therefore did not reduce Abeta burden or improve cognition. Significantly, the immunotherapy in the 11-24 months treatment group, that reduced Abeta burden, did not increase cerebral bleeding or vascular Abeta deposits in contrast to several Abeta antibody studies. These findings indicate that our approach age-dependently improves cognition and reduces Abeta burden when used with an adjuvant suitable for humans, without increasing vascular Abeta deposits or microhemorrhages.
Figures


References
-
- Acarin L, Vela JM, Gonzalez B, Castellano B. Demonstration of poly--acetyl lactosamine residues in ameboid and ramified microglial cells in rat brain by tomato lectin binding. J Histochem Cytochem. 1994;42:1033–1041. - PubMed
-
- Agadjanyan MG, Ghochikyan A, Petrushina I, Vasilevko V, Movsesyan N, Mkrtichyan M, Saing T, Cribbs DH. Prototype Alzheimer’s disease vaccine using the immunodominant B cell epitope from β-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J Immunol. 2005;174:1580–1586. - PubMed
-
- Bennett DA, Holtzman DM. Immunization therapy for Alzheimer disease? Neurology. 2005;64:10–12. - PubMed
-
- Chauhan NB, Siegel GJ. Intracerebroventricular passive immunization with anti-Aβ antibody in Tg2576. J Neurosci Res. 2003;74:142–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

