Activation, coactivation, and costimulation of resting human natural killer cells
- PMID: 17100877
- PMCID: PMC3845883
- DOI: 10.1111/j.1600-065X.2006.00457.x
Activation, coactivation, and costimulation of resting human natural killer cells
Abstract
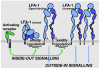
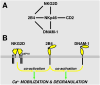
Natural killer (NK) cells possess potent perforin- and interferon-gamma-dependent effector functions that are tightly regulated. Inhibitory receptors for major histocompatibility complex class I display variegated expression among NK cells, which confers specificity to individual NK cells. Specificity is also provided by engagement of an array of NK cell activation receptors. Target cells may express ligands for a multitude of activation receptors, many of which signal through different pathways. How inhibitory receptors intersect different signaling cascades is not fully understood. This review focuses on advances in understanding how activation receptors cooperate to induce cytotoxicity in resting NK cells. The role of activating receptors in determining specificity and providing redundancy of target cell recognition is discussed. Using Drosophila insect cells as targets, we have examined the contribution of individual receptors. Interestingly, the strength of activation is not determined simply by additive effects of parallel activation pathways. Combinations of signals from different receptors can have different outcomes: synergy, no enhancement over individual signals, or additive effects. Cytotoxicity requires combined signals for granule polarization and degranulation. The integrin leukocyte function-associated antigen-1 contributes a signal for polarization but not for degranulation. Conversely, CD16 alone or in synergistic combinations, such as NKG2D and 2B4, signals for phospholipase-C-gamma- and phosphatidylinositol-3-kinase-dependent degranulation.
Figures







References
-
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. - PubMed
-
- Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–747. - PubMed
-
- Bossi G, Griffiths GM. CTL secretory lysosomes: biogenesis and secretion of a harmful organelle. Semin Immunol. 2005;17:87–94. - PubMed
-
- O'Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol. 2002;2:37–45. - PubMed
-
- Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

