T-cell contact-dependent regulation of CC and CXC chemokine production in monocytes through differential involvement of NFkappaB: implications for rheumatoid arthritis
- PMID: 17101049
- PMCID: PMC1794512
- DOI: 10.1186/ar2077
T-cell contact-dependent regulation of CC and CXC chemokine production in monocytes through differential involvement of NFkappaB: implications for rheumatoid arthritis
Abstract
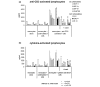
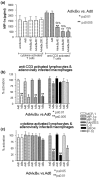
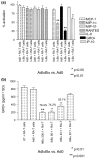
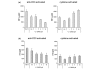
We and others have reported that rheumatoid arthritis (RA) synovial T cells can activate human monocytes/macrophages in a contact-dependent manner to induce the expression of inflammatory cytokines, including tumour necrosis factor alpha (TNFalpha). In the present study we demonstrate that RA synovial T cells without further activation can also induce monocyte CC and CXC chemokine production in a contact-dependent manner. The transcription factor NFkappaB is differentially involved in this process as CXC chemokines but not CC chemokines are inhibited after overexpression of IkappaBalpha, the natural inhibitor of NFkappaB. This effector function of RA synovial T cells is also shared by T cells activated with a cytokine cocktail containing IL-2, IL-6 and TNFalpha, but not T cells activated by anti-CD3 cross-linking that mimics TCR engagement. This study demonstrates for the first time that RA synovial T cells as well as cytokine-activated T cells are able to induce monocyte chemokine production in a contact-dependent manner and through NFkappaB-dependent and NFkappaB-independent mechanisms, in a process influenced by the phosphatidyl-inositol-3-kinase pathway. Moreover, this study provides further evidence that cytokine-activated T cells share aspects of their effector function with RA synovial T cells and that their targeting in the clinic has therapeutic potential.
Figures
Similar articles
-
Interleukin-18 enhances monocyte tumor necrosis factor alpha and interleukin-1beta production induced by direct contact with T lymphocytes: implications in rheumatoid arthritis.Arthritis Rheum. 2004 Feb;50(2):432-43. doi: 10.1002/art.20064. Arthritis Rheum. 2004. PMID: 14872485
-
Cytokine stimulation of T lymphocytes regulates their capacity to induce monocyte production of tumor necrosis factor-alpha, but not interleukin-10: possible relevance to pathophysiology of rheumatoid arthritis.Eur J Immunol. 1997 Mar;27(3):624-32. doi: 10.1002/eji.1830270308. Eur J Immunol. 1997. PMID: 9079801
-
Interleukin-18 induces angiogenic factors in rheumatoid arthritis synovial tissue fibroblasts via distinct signaling pathways.Arthritis Rheum. 2007 Jun;56(6):1787-97. doi: 10.1002/art.22705. Arthritis Rheum. 2007. PMID: 17530707
-
Molecular aspects of rheumatoid arthritis: chemokines in the joints of patients.FEBS J. 2008 Sep;275(18):4448-55. doi: 10.1111/j.1742-4658.2008.06580.x. Epub 2008 Jul 24. FEBS J. 2008. PMID: 18662305 Review.
-
The autoimmune pathogenesis of rheumatoid arthritis: role of autoreactive T cells and new immunotherapies.Semin Arthritis Rheum. 2001 Dec;31(3):160-75. doi: 10.1053/sarh.2001.27736. Semin Arthritis Rheum. 2001. PMID: 11740797 Review.
Cited by
-
Leukocyte-specific protein 1 regulates T-cell migration in rheumatoid arthritis.Proc Natl Acad Sci U S A. 2015 Nov 24;112(47):E6535-43. doi: 10.1073/pnas.1514152112. Epub 2015 Nov 9. Proc Natl Acad Sci U S A. 2015. PMID: 26554018 Free PMC article.
-
Developments in the scientific understanding of rheumatoid arthritis.Arthritis Res Ther. 2009;11(5):249. doi: 10.1186/ar2758. Epub 2009 Oct 14. Arthritis Res Ther. 2009. PMID: 19835638 Free PMC article. Review.
-
Efficacy of Bioactive Cyclic Peptides in Rheumatoid Arthritis: Translation from In Vitro to In Vivo Models.Molecules. 2017 Sep 25;22(10):1613. doi: 10.3390/molecules22101613. Molecules. 2017. PMID: 28946707 Free PMC article.
-
Design and optimisation of bioactive cyclic peptides: generation of a down-regulator of TNF secretion.Molecules. 2014 Dec 22;19(12):21529-40. doi: 10.3390/molecules191221529. Molecules. 2014. PMID: 25532847 Free PMC article.
-
Alpha-1-antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood.J Leukoc Biol. 2009 May;85(5):886-95. doi: 10.1189/jlb.0208145. Epub 2009 Feb 5. J Leukoc Biol. 2009. PMID: 19197072 Free PMC article.
References
-
- Brennan FM, Zachariae CO, Chantry D, Larsen CG, Turner M, Maini RN, Matsushima K, Feldmann M. Detection of interleukin 8 biological activity in synovial fluids from patients with rheumatoid arthritis and production of interleukin 8 mRNA by isolated synovial cells. Eur J Immunol. 1990;20:2141–2144. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

