Structural and mechanistic features of protein O glycosylation linked to CD8+ T-cell apoptosis
- PMID: 17101770
- PMCID: PMC1800694
- DOI: 10.1128/MCB.01750-06
Structural and mechanistic features of protein O glycosylation linked to CD8+ T-cell apoptosis
Abstract
CD8+ T-cell apoptosis is essential for the contraction phase of the immune response, yet the initiating signals and precise pathways involved are unresolved. The ST3Gal-I sialyltransferase is a candidate mechanistic component and catalyzes sialic acid addition to core 1 O-glycans during protein O glycosylation. ST3Gal-I inactivation or enzymatic removal of its product renders CD8+ T cells, but not CD4+ T cells, susceptible to apoptosis by differential cross-linking of O-glycoproteins in the absence of interleukin-2 and T-cell receptor (TCR) signaling. This results in caspase activation, DNA fragmentation, and phosphatidylserine externalization prior to cell death. We further show that ST3Gal-I function is regulated by a posttranscriptional mechanism operating distal to Golgi core 2 O glycosylation and is invariably linked to CD8+ T-cell contraction following viral (lymphocytic choriomeningitis virus) infection and bacterial (staphylococcal enterotoxin B) antigen immunization. The mechanism does not involve the ST3Gal-I substrate CD43 or core 2 O-glycan induction and overcomes the ability of Bcl-2 to inhibit the contraction phase in vivo. Loss of ST3Gal-I function further reduces Bim-deficient CD8+ T-cell accumulation without diminishing apoptotic sensitivity. We propose that an endogenous lectin activates an apoptotic pathway constructed in CD8+ T cells following TCR stimulation and enables contraction upon attenuation of immune signaling.
Figures
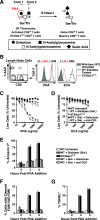

 , statistically significant difference from wild-type littermates (P < 0.05, paired t test).
, statistically significant difference from wild-type littermates (P < 0.05, paired t test).


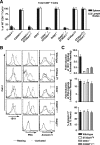
 , statistically significant difference from wild-type littermates (P < 0.05, unpaired t test).
, statistically significant difference from wild-type littermates (P < 0.05, unpaired t test).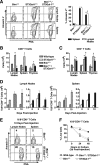
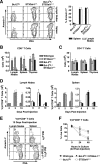
 , statistically significant difference from ST3Gal-IΔ/Δ (in panel A) or wild-type littermates (in panels B and C) (P < 0.05, unpaired t test).
, statistically significant difference from ST3Gal-IΔ/Δ (in panel A) or wild-type littermates (in panels B and C) (P < 0.05, unpaired t test).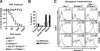

Similar articles
-
The ST3Gal-I sialyltransferase controls CD8+ T lymphocyte homeostasis by modulating O-glycan biosynthesis.Immunity. 2000 Mar;12(3):273-83. doi: 10.1016/s1074-7613(00)80180-6. Immunity. 2000. PMID: 10755614
-
Developmentally regulated glycosylation of the CD8alphabeta coreceptor stalk modulates ligand binding.Cell. 2001 Nov 16;107(4):501-12. doi: 10.1016/s0092-8674(01)00577-3. Cell. 2001. PMID: 11719190
-
The sialyltransferase ST3Gal-I is not required for regulation of CD8-class I MHC binding during T cell development.J Immunol. 2006 Jun 15;176(12):7421-30. doi: 10.4049/jimmunol.176.12.7421. J Immunol. 2006. PMID: 16751387
-
Genetically altered mice with different sialyltransferase deficiencies show tissue-specific alterations in sialylation and sialic acid 9-O-acetylation.J Biol Chem. 2002 Sep 6;277(36):32930-8. doi: 10.1074/jbc.M203362200. Epub 2002 Jun 14. J Biol Chem. 2002. PMID: 12068010
-
Contracting the 'mus cells'--does down-sizing suit us for diving into the memory pool?Immunol Rev. 2010 Jul;236:54-67. doi: 10.1111/j.1600-065X.2010.00920.x. Immunol Rev. 2010. PMID: 20636808 Free PMC article. Review.
Cited by
-
Mass spectrometry-based identification of the tumor antigen UN1 as the transmembrane CD43 sialoglycoprotein.Mol Cell Proteomics. 2011 May;10(5):M111.007898. doi: 10.1074/mcp.M111.007898. Epub 2011 Mar 3. Mol Cell Proteomics. 2011. PMID: 21372249 Free PMC article.
-
Identification of STAT5A and STAT5B target genes in human T cells.PLoS One. 2014 Jan 30;9(1):e86790. doi: 10.1371/journal.pone.0086790. eCollection 2014. PLoS One. 2014. PMID: 24497979 Free PMC article.
-
Initiation of protein O glycosylation by the polypeptide GalNAcT-1 in vascular biology and humoral immunity.Mol Cell Biol. 2007 Dec;27(24):8783-96. doi: 10.1128/MCB.01204-07. Epub 2007 Oct 8. Mol Cell Biol. 2007. PMID: 17923703 Free PMC article.
-
Regulation of T Cell Trafficking by Enzymatic Synthesis of O-Glycans.Front Immunol. 2017 May 24;8:600. doi: 10.3389/fimmu.2017.00600. eCollection 2017. Front Immunol. 2017. PMID: 28596771 Free PMC article. Review.
-
Genome-wide differential gene expression in immortalized DF-1 chicken embryo fibroblast cell line.BMC Genomics. 2011 Nov 23;12:571. doi: 10.1186/1471-2164-12-571. BMC Genomics. 2011. PMID: 22111699 Free PMC article.
References
-
- Amado, M., Q. Yan, E. M. Comelli, B. E. Collins, and J. C. Paulson. 2004. Peanut agglutinin high phenotype of activated CD8+ T cells results from de novo synthesis of CD45 glycans. J. Biol. Chem. 279:36689-36697. - PubMed
-
- Badovinac, V. P., A. R. Tvinnereim, and J. T. Harty. 2000. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-γ. Science 290:1354-1357. - PubMed
-
- Badovinac, V. P., B. B. Porter, and J. T. Harty. 2004. CD8+ T cell contraction is controlled by early inflammation. Nat. Immunol. 5:809-817. - PubMed
-
- Bouillet, P., D. Metcalf, D. C. S. Huang, D. M. Tarlington, T. W. H. Kay, F. Köntgen, J. M. Adams, and A. Strasser. 1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286:1735-1738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
