X-ray fused with magnetic resonance imaging (XFM) to target endomyocardial injections: validation in a swine model of myocardial infarction
- PMID: 17101858
- PMCID: PMC2020803
- DOI: 10.1161/CIRCULATIONAHA.105.598524
X-ray fused with magnetic resonance imaging (XFM) to target endomyocardial injections: validation in a swine model of myocardial infarction
Abstract
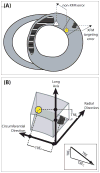
Background: Magnetic resonance imaging (MRI) permits 3-dimensional (3D) cardiac imaging with high soft tissue contrast. X-ray fluoroscopy provides high-resolution, 2-dimensional (2D) projection imaging. We have developed real-time x-ray fused with MRI (XFM) to guide invasive procedures that combines the best features of both imaging modalities. We tested the accuracy of XFM using external fiducial markers to guide endomyocardial cell injections in infarcted swine hearts.
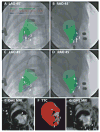
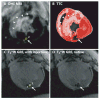
Methods and results: Endomyocardial injections of iron-labeled mesenchymal stromal cells admixed with tissue dye were performed in previously infarcted hearts of 12 Yucatan miniswine (weight, 33 to 67 kg). Features from cardiac MRI were displayed combined with x-ray in real time to guide injections. During 130 injections, operators were provided with 3D surfaces of endocardium, epicardium, myocardial wall thickness (range, 2.6 to 17.7 mm), and infarct registered with live x-ray images to facilitate device navigation and choice of injection location. XFM-guided injections were compared with postinjection MRI and with necropsy specimens obtained 24 hours later. Visual inspection of the pattern of dye staining on 2,3,5-triphenyltetrazolium chloride-stained heart slices agreed (kappa=0.69) with XFM-derived injection locations mapped onto delayed hyperenhancement MRI and the susceptibility artifacts seen on the postinjection T2*-weighted gradient echo MRI. The distance between the predicted and actual injection locations in vivo was 3.2+/-2.6 mm (n=64), and 75% of injections were within 4.1 mm of the predicted location.
Conclusions: Three-dimensional to two-dimensional registration of x-ray and MR images with the use of external fiducial markers accurately targets endomyocardial injection in a swine model of myocardial infarction.
Figures

References
-
- Dohmann HF, Perin EC, Takiya CM, Silva GV, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Pascarelli BM, Assis IM, Dutra HS, Assad JA, Castello-Branco RV, Drummond C, Dohmann HJ, Willerson JT, Borojevic R. Transendocardial autologous bone marrow mononuclear cell injection in ischemic heart failure: postmortem anatomicopathologic and immunohistochemical findings. Circulation. 2005;112:521–526. - PubMed
-
- Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, Hanley A, Silver M, Kearney M, Losordo DW, Isner JM, Asahara T. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neo-vascularization of myocardial ischemia. Circulation. 2003;107:461–468. - PubMed
-
- Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Carvalho AC, Dutra HS, Dohmann HJ, Silva GV, Belem L, Vivacqua R, Rangel FO, Esporcatte R, Geng YJ, Vaughn WK, Assad JA, Mesquita ET, Willerson JT. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. - PubMed
-
- Chazaud B, Hittinger L, Sonnet C, Champagne S, Le Corvoisier P, Benhaiem-Sigaux N, Unterseeh T, Su J, Merlet P, Rahmouni A, Garot J, Gherardi R, Teiger E. Endoventricular porcine autologous myoblast transplantation can be successfully achieved with minor mechanical cell damage. Cardiovasc Res. 2003;58:444–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

