Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel
- PMID: 17101973
- PMCID: PMC1838753
- DOI: 10.1073/pnas.0604073103
Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel
Abstract
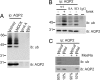
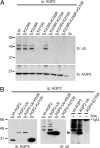
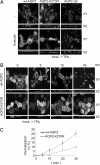
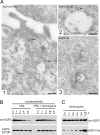
To regulate mammalian water homeostasis, arginine-vasopressin (AVP) induces phosphorylation and thereby redistribution of renal aquaporin-2 (AQP2) water channels from vesicles to the apical membrane. Vice versa, AVP (or forskolin) removal and hormones activating PKC cause AQP2 internalization, but the mechanism is unknown. Here, we show that a fraction of AQP2 is modified with two to three ubiquitin moieties in vitro and in vivo. Mutagenesis revealed that AQP2 is ubiquitinated with one K63-linked chain at K270 only. In Madin-Darby canine kidney cells, AQP2 ubiquitination occurs preferentially when present in the apical membrane, is transiently increased with forskolin removal or PKC activation, and precedes its internalization. Internalization kinetics assays with wild type (wt) and ubiquitination-deficient (K270R) AQP2 revealed that ubiquitination enhances AQP2 endocytosis. Electron microscopy showed that a translational fusion of AQP2 with ubiquitin (AQP2-Ub) localized particularly to internal vesicles of multivesicular bodies (MVBs), whereas AQP2-K270R largely localized to the apical membrane, early endosomes, and the limiting membrane of MVBs. Consistent with this distribution pattern, lysosomal degradation was extensive for AQP2-Ub, low for AQP2-K270R, and intermediate for wt-AQP2. Our data show that short-chain ubiquitination is involved in the regulated endocytosis, MVB sorting, and degradation of AQP2 and may be the mechanism used by AVP removal and PKC-activating hormones to reduce renal water reabsorption. Moreover, because several other channels are also (short-chain) ubiquitinated, our data suggest that ubiquitination may be a general mediator for the regulated endocytosis and degradation of channels in higher eukaryotes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

