CARMA3/Bcl10/MALT1-dependent NF-kappaB activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells
- PMID: 17101977
- PMCID: PMC1766317
- DOI: 10.1073/pnas.0601947103
CARMA3/Bcl10/MALT1-dependent NF-kappaB activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells
Abstract
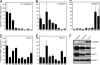
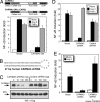
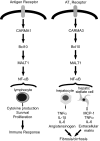
Angiotensin II (Ang II) is a peptide hormone that, like many cytokines, acts as a proinflammatory agent and growth factor. After injury to the liver, the hormone assists in tissue repair by stimulating hepatocytes and hepatic stellate cells to synthesize extracellular matrix proteins and secrete secondary cytokines and by stimulating myofibroblasts to proliferate. However, under conditions of chronic liver injury, all of these effects conspire to promote pathologic liver fibrosis. Much of this effect of Ang II results from activation of the proinflammatory NF-kappaB transcription factor in response to stimulation of the type 1 Ang II receptor, a G protein-coupled receptor. Here, we characterize a previously undescribed signaling pathway mediating Ang II-dependent activation of NF-kappaB, which is composed of three principal proteins, CARMA3, Bcl10, and MALT1. Blocking the function of any of these proteins, through the use of either dominant-negative mutants, RNAi, or gene targeting, effectively abolishes Ang II-dependent NF-kappaB activation in hepatocytes. In addition, Bcl10(-/-) mice show defective hepatic cytokine production after Ang II treatment. Evidence also is presented that this pathway activates NF-kappaB through ubiquitination of IKKgamma, the regulatory subunit of the IkappaB kinase complex. These results elucidate a concrete series of molecular events that link ligand activation of the type 1 Ang II receptor to stimulation of the NF-kappaB transcription factor. These findings also uncover a function of the CARMA, Bcl10, and MALT1 proteins in cells outside the immune system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Phillips MI, Kagiyama S. Curr Opin Investig Drugs. 2002;3:569–577. - PubMed
-
- Bataller R, Sancho-Bru P, Gines P, Brenner DA. Antioxid Redox Signal. 2005;7:1346–1355. - PubMed
-
- Bataller R, Gines P, Nicolas JM, Gorbig MN, Garcia-Ramallo E, Gasull X, Bosch J, Arroyo V, Rodes J. Gastroenterology. 2000;118:1149–1156. - PubMed
-
- Shah BH, Alberto Olivares-Reyes J, Yesilkaya A, Catt KJ. Mol Endocrinol. 2002;16:610–620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

