Insulin-like growth factor factor binding protein-2 is a novel mediator of p53 inhibition of insulin-like growth factor signaling
- PMID: 17102589
- PMCID: PMC1906874
- DOI: 10.4161/cbt.5.10.3455
Insulin-like growth factor factor binding protein-2 is a novel mediator of p53 inhibition of insulin-like growth factor signaling
Abstract
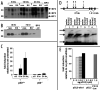
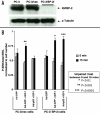
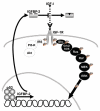
The p53 tumor suppressor induces cellular growth arrest and apoptosis in response to DNA damage by transcriptionally activating or repressing target genes and also through protein-protein interactions and direct mitochondrial activities. In 1995, insulin-like growth factor binding protein (IGFBP)-3 was identified as one of the genes transcriptionally activated by p53. IGFBP-3 is one of six closely related IGFBP's, with additional IGFBP-related proteins belonging to the IGFBP superfamily. Here we show that IGFBP-2 is also a p53 target. Like IGFBP-3, IGFBP-2 secretion is reduced when p53+/+ lung cancer cells are transfected with human papillomavirus E6, which targets p53 for degradation. IGFBP-2 mRNA is induced by irradiation in vivo in a p53-dependent manner. p53 protein binds IGFBP-2 intronic sequences in an electrophoretic mobility shift assay, and activates transcription in a luciferase assay. Loss of IGFBP-2 inhibits the ability of p53 to inhibit the activation of extracellular signal-regulated kinase (ERK)1 by IGF-I. Thus, p53 effects on the IGF axis are more complex than previously appreciated, and overall transform the axis from IGF-mediated mitogenesis to growth inhibition and apoptosis. This has significant implications for how growth hormone and IGF-I can induce growth without also inducing cancer.
Figures
Similar articles
-
Insulin-like growth factor-binding protein-3 suppresses tumor growth via activation of caspase-dependent apoptosis and cross-talk with NF-κB signaling.Cancer Lett. 2011 Aug 28;307(2):200-10. doi: 10.1016/j.canlet.2011.04.004. Epub 2011 May 4. Cancer Lett. 2011. PMID: 21536375
-
P53 and IGFBP-3: apoptosis and cancer protection.Mol Genet Metab. 2000 Jun;70(2):85-98. doi: 10.1006/mgme.2000.3008. Mol Genet Metab. 2000. PMID: 10873390 Review.
-
Evidence for a role for insulin-like growth factor binding proteins in glucocorticoid inhibition of normal human osteoblast-like cell proliferation.Eur J Endocrinol. 1996 May;134(5):591-601. doi: 10.1530/eje.0.1340591. Eur J Endocrinol. 1996. PMID: 8664980
-
Elevated insulin-like growth factor (IGF) binding protein (IGFBP)-2 and IGFBP-4 expression of leukemic T-cells is affected by autocrine/paracrine IGF-II action but not by IGF type I receptor expression.Eur J Endocrinol. 1998 Mar;138(3):337-43. doi: 10.1530/eje.0.1380337. Eur J Endocrinol. 1998. PMID: 9539310
-
Cellular actions of the insulin-like growth factor binding proteins.Endocr Rev. 2002 Dec;23(6):824-54. doi: 10.1210/er.2001-0033. Endocr Rev. 2002. PMID: 12466191 Review.
Cited by
-
Involvement of transcription factor p53 and leptin in control of porcine ovarian granulosa cell functions.Cell Prolif. 2012 Feb;45(1):9-14. doi: 10.1111/j.1365-2184.2011.00793.x. Epub 2011 Dec 7. Cell Prolif. 2012. PMID: 22151798 Free PMC article.
-
Silencing IGFBP-2 decreases pancreatic cancer metastasis and enhances chemotherapeutic sensitivity.Oncotarget. 2017 Jun 27;8(37):61674-61686. doi: 10.18632/oncotarget.18669. eCollection 2017 Sep 22. Oncotarget. 2017. PMID: 28977895 Free PMC article.
-
MDA-9/syntenin and IGFBP-2 promote angiogenesis in human melanoma.Cancer Res. 2013 Jan 15;73(2):844-54. doi: 10.1158/0008-5472.CAN-12-1681. Epub 2012 Dec 10. Cancer Res. 2013. PMID: 23233738 Free PMC article.
-
Role of insulin-like growth factor binding protein 2 in lung adenocarcinoma: IGF-independent antiapoptotic effect via caspase-3.Am J Pathol. 2010 Apr;176(4):1756-66. doi: 10.2353/ajpath.2010.090500. Epub 2010 Feb 11. Am J Pathol. 2010. PMID: 20150439 Free PMC article.
-
Igf2 pathway dependency of the Trp53 developmental and tumour phenotypes.EMBO Mol Med. 2012 Aug;4(8):705-18. doi: 10.1002/emmm.201101105. Epub 2012 Jun 6. EMBO Mol Med. 2012. PMID: 22674894 Free PMC article.
References
-
- Guimaraes DP, Hainaut P. TP53: A key gene in human cancer. Biochimie. 2002;84:83–93. - PubMed
-
- Varley JM. Germline TP53 mutations and Li-Fraumeni syndrome. Human Mutn. 2003;21:313–20. - PubMed
-
- Parant JM, Lozano G. Disrupting TP53 in mouse models of human cancers. Human Mutn. 2003;21:321–6. - PubMed
-
- Giono LE, Manfredi JJ. The p53 tumor suppressor participates in multiple cell cycle checkpoints. J Cell Physiol. 2006;209:13–20. - PubMed
-
- Oren M. Decision making by p53: Life, death and cancer. Cell Death Differ. 2003;10:431–442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
