Deuterium isotope effects in the unusual addition of toluene to fumarate catalyzed by benzylsuccinate synthase
- PMID: 17105211
- PMCID: PMC2519130
- DOI: 10.1021/bi061117o
Deuterium isotope effects in the unusual addition of toluene to fumarate catalyzed by benzylsuccinate synthase
Abstract
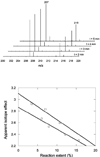
The first step in the anaerobic metabolism of toluene is a highly unusual reaction: the addition of toluene across the double bond of fumarate to produce (R)-benzylsuccinate, which is catalyzed by benzylsuccinate synthase. Benzylsuccinate synthase is a member of the glycyl radical-containing family of enzymes, and the reaction is initiated by abstraction of a hydrogen atom from the methyl group of toluene. To gain insight into the free energy profile of this reaction, we have measured the kinetic isotope effects on Vmax and Vmax/Km when deuterated toluene is the substrate. At 30 degrees C the isotope effects are 1.7 +/- 0.2 and 2.9 +/- 0.1 on Vmax and Vmax/Km, respectively; at 4 degrees C they increase slightly to 2.2 +/- 0.2 and 3.1 +/- 0.1, respectively. We compare these results with the theoretical isotope effects on Vmax and Vmax/Km that are predicted from the free energy profile for the uncatalyzed reaction, which has previously been computed using density functional theory [Himo, F. (2002) J. Phys. Chem. B 106, 7688-7692]. The comparison allows us to draw some conclusions on how the enzyme may catalyze this unusual reaction.
Figures




References
-
- Spormann AM, Widdel F. Metabolism of alkylbenzenes, alkanes, and other hydrocarbons in anaerobic bacteria. Biodegradation. 2000;11:85–105. - PubMed
-
- Heider J, Spormann AM, Beller HR, Widdel F. Anaerobic bacterial metabolism of hydrocarbons. Fems Microbiol. Rev. 1998;22:459–473.
-
- Biegert T, Fuchs G, Heider F. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur. J. Biochem. 1996;238:661–668. - PubMed
-
- Rabus R, Heider J. Initial reactions of anaerobic metabolism of alkylbenzenes in denitrifying and sulfate reducing bacteria. Arch. Microbiol. 1998;170:377–384.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

