The genome of deep-sea vent chemolithoautotroph Thiomicrospira crunogena XCL-2
- PMID: 17105352
- PMCID: PMC1635747
- DOI: 10.1371/journal.pbio.0040383
The genome of deep-sea vent chemolithoautotroph Thiomicrospira crunogena XCL-2
Abstract
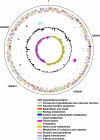
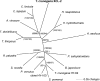
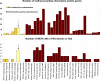
Presented here is the complete genome sequence of Thiomicrospira crunogena XCL-2, representative of ubiquitous chemolithoautotrophic sulfur-oxidizing bacteria isolated from deep-sea hydrothermal vents. This gammaproteobacterium has a single chromosome (2,427,734 base pairs), and its genome illustrates many of the adaptations that have enabled it to thrive at vents globally. It has 14 methyl-accepting chemotaxis protein genes, including four that may assist in positioning it in the redoxcline. A relative abundance of coding sequences (CDSs) encoding regulatory proteins likely control the expression of genes encoding carboxysomes, multiple dissolved inorganic nitrogen and phosphate transporters, as well as a phosphonate operon, which provide this species with a variety of options for acquiring these substrates from the environment. Thiom. crunogena XCL-2 is unusual among obligate sulfur-oxidizing bacteria in relying on the Sox system for the oxidation of reduced sulfur compounds. The genome has characteristics consistent with an obligately chemolithoautotrophic lifestyle, including few transporters predicted to have organic allocrits, and Calvin-Benson-Bassham cycle CDSs scattered throughout the genome.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures






Comment in
-
Genomic insights into (extreme) life at the bottom of the sea.PLoS Biol. 2006 Dec;4(12):e425. doi: 10.1371/journal.pbio.0040425. Epub 2006 Nov 14. PLoS Biol. 2006. PMID: 20076510 Free PMC article. No abstract available.
Similar articles
-
Proteomic and Mutant Analysis of the CO2 Concentrating Mechanism of Hydrothermal Vent Chemolithoautotroph Thiomicrospira crunogena.J Bacteriol. 2017 Mar 14;199(7):e00871-16. doi: 10.1128/JB.00871-16. Print 2017 Apr 1. J Bacteriol. 2017. PMID: 28115547 Free PMC article.
-
Dissolved inorganic carbon uptake in Thiomicrospira crunogena XCL-2 is Δp- and ATP-sensitive and enhances RubisCO-mediated carbon fixation.Arch Microbiol. 2016 Mar;198(2):149-59. doi: 10.1007/s00203-015-1172-6. Epub 2015 Nov 18. Arch Microbiol. 2016. PMID: 26581415
-
Metagenomic comparison of two Thiomicrospira lineages inhabiting contrasting deep-sea hydrothermal environments.PLoS One. 2010 Oct 21;5(10):e13530. doi: 10.1371/journal.pone.0013530. PLoS One. 2010. PMID: 20975831 Free PMC article.
-
Occurrence, phylogeny and evolution of ribulose-1,5-bisphosphate carboxylase/oxygenase genes in obligately chemolithoautotrophic sulfur-oxidizing bacteria of the genera Thiomicrospira and Thioalkalimicrobium.Microbiology (Reading). 2006 Jul;152(Pt 7):2159-2169. doi: 10.1099/mic.0.28699-0. Microbiology (Reading). 2006. PMID: 16804189
-
Diversity in CO2-Concentrating Mechanisms among Chemolithoautotrophs from the Genera Hydrogenovibrio, Thiomicrorhabdus, and Thiomicrospira, Ubiquitous in Sulfidic Habitats Worldwide.Appl Environ Microbiol. 2019 Jan 23;85(3):e02096-18. doi: 10.1128/AEM.02096-18. Print 2019 Feb 1. Appl Environ Microbiol. 2019. PMID: 30446552 Free PMC article.
Cited by
-
Kinetic enrichment of 34S during proteobacterial thiosulfate oxidation and the conserved role of SoxB in S-S bond breaking.Appl Environ Microbiol. 2013 Jul;79(14):4455-64. doi: 10.1128/AEM.00956-13. Epub 2013 May 17. Appl Environ Microbiol. 2013. PMID: 23686269 Free PMC article.
-
Sulfur and Methane-Oxidizing Microbial Community in a Terrestrial Mud Volcano Revealed by Metagenomics.Microorganisms. 2020 Aug 31;8(9):1333. doi: 10.3390/microorganisms8091333. Microorganisms. 2020. PMID: 32878336 Free PMC article.
-
Bacterial lifestyle in a deep-sea hydrothermal vent chimney revealed by the genome sequence of the thermophilic bacterium Deferribacter desulfuricans SSM1.DNA Res. 2010 Jun;17(3):123-37. doi: 10.1093/dnares/dsq005. Epub 2010 Feb 26. DNA Res. 2010. PMID: 20189949 Free PMC article.
-
The temperate marine phage PhiHAP-1 of Halomonas aquamarina possesses a linear plasmid-like prophage genome.J Virol. 2008 Jul;82(13):6618-30. doi: 10.1128/JVI.00140-08. Epub 2008 Apr 30. J Virol. 2008. PMID: 18448537 Free PMC article.
-
Cultivation-Independent and Cultivation-Dependent Analysis of Microbes in the Shallow-Sea Hydrothermal System Off Kueishantao Island, Taiwan: Unmasking Heterotrophic Bacterial Diversity and Functional Capacity.Front Microbiol. 2018 Feb 22;9:279. doi: 10.3389/fmicb.2018.00279. eCollection 2018. Front Microbiol. 2018. PMID: 29527196 Free PMC article.
References
-
- Karl DM, Wirsen CO, Jannasch HW. Deep-sea primary production at the Galápagos hydrothermal vents. Science. 1980;207:1345–1346.
-
- Kelley DS, Karson JA, Fruh-Green GL, Yoerger DR, Shank TM, et al. A serpentinite-hosted ecosystem: The lost city hydrothermal field. Science. 2005;307:1428–1434. - PubMed
-
- Johnson KS, Childress JJ, Beehler CL. Short term temperature variability in the Rose Garden hydrothermal vent field. Deep-Sea Res. 1988;35:1711–1722.
-
- Goffredi SK, Childress JJ, Desaulniers NT, Lee RW, Lallier FH, et al. Inorganic carbon acquisition by the hydrothermal vent tubeworm Riftia pachyptila depends upon high external P-CO2 and upon proton-equivalent ion transport by the worm. J Exp Biol. 1997;200:883–896. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

