Effects of serial levosimendan infusions on left ventricular performance and plasma biomarkers of myocardial injury and neurohormonal and immune activation in patients with advanced heart failure
- PMID: 17105880
- PMCID: PMC1861282
- DOI: 10.1136/hrt.2006.079707
Effects of serial levosimendan infusions on left ventricular performance and plasma biomarkers of myocardial injury and neurohormonal and immune activation in patients with advanced heart failure
Erratum in
- Heart. 2009 Jan;95(1):84
Abstract
Background: Levosimendan is a novel inodilator that improves central haemodynamics and symptoms of patients with decompensated chronic heart failure. The role, however, of repeated levosimendan infusions in the management of these patients has not yet been properly assessed.
Purpose: This randomised placebo-controlled trial investigated the effects of serial levosimendan infusions on cardiac geometry and function, and on biomarkers of myocardial injury and neurohormonal and immune activation (troponin T, N-terminal B-type natriuretic pro-peptide (NT-proBNP), C reactive protein (CRP) and interleukin (IL) 6) in patients with advanced heart failure.
Methods: 25 patients with decompensated chronic heart failure were randomised (2:1) to receive five serial 24-h infusions (every 3 weeks) of either levosimendan (n = 17) or placebo (n = 8), and were evaluated echocardiographically and biochemically before and after each drug infusion and 30 days after the final infusion.
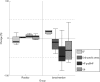
Results: Following treatment, cardiac end-systolic and end-diastolic dimension and volume indices were significantly reduced only in the levosimendan-treated patients (p<0.01). A significant decrease in NT-proBNP (p<0.01), high-sensitivity CRP (p<0.01) and plasma IL6 (p = 0.05) was also observed in the levosimendan group, whereas these markers remained unchanged in the placebo group; similar changes were observed after each drug infusion. Although the number of patients with a positive troponin T (>or=0.01 ng/ml) was not different between the two groups at baseline, it was significantly higher in the placebo-treated group during the final evaluation (p<0.05).
Conclusion: Serial levosimendan treatments improved left ventricular performance and modulated neurohormonal and immune activation beneficially in patients with advanced heart failure, without increasing myocardial injury.
Conflict of interest statement
Competing interests: None declared.
Similar articles
-
Assessment of sustained effects of levosimendan and dobutamine on left ventricular systolic functions by using novel tissue Doppler derived indices in patients with advanced heart failure.Cardiol J. 2015;22(1):87-93. doi: 10.5603/CJ.a2014.0044. Epub 2014 May 20. Cardiol J. 2015. PMID: 24846511 Clinical Trial.
-
The predictive value of QRS duration in response to levosimendan therapy in patients with decompensated heart failure.Acta Cardiol. 2012 Jun;67(3):317-23. doi: 10.1080/ac.67.3.2160721. Acta Cardiol. 2012. PMID: 22870740
-
Effects of levosimendan on markers of left ventricular diastolic function and neurohormonal activation in patients with advanced heart failure.Am J Cardiol. 2005 Aug 1;96(3):423-6. doi: 10.1016/j.amjcard.2005.03.092. Am J Cardiol. 2005. PMID: 16054474 Clinical Trial.
-
Levosimendan: first in a new class of inodilator for acute and chronic severe heart failure.Expert Rev Cardiovasc Ther. 2004 Jan;2(1):9-19. doi: 10.1586/14779072.2.1.9. Expert Rev Cardiovasc Ther. 2004. PMID: 15038409 Review.
-
Repetitive use of levosimendan in advanced heart failure: need for stronger evidence in a field in dire need of a useful therapy.Int J Cardiol. 2017 Sep 15;243:389-395. doi: 10.1016/j.ijcard.2017.05.081. Epub 2017 May 23. Int J Cardiol. 2017. PMID: 28571618 Review.
Cited by
-
Tuning cardiac performance in ischemic heart disease and failure by modulating myofilament function.J Mol Med (Berl). 2007 Sep;85(9):911-21. doi: 10.1007/s00109-007-0181-6. Epub 2007 Mar 30. J Mol Med (Berl). 2007. PMID: 17396243 Review.
-
Single-center experience with levosimendan in children undergoing cardiac surgery and in children with decompensated heart failure.BMC Anesthesiol. 2011 Oct 5;11:18. doi: 10.1186/1471-2253-11-18. BMC Anesthesiol. 2011. PMID: 21974814 Free PMC article.
-
Beneficial impact of levosimendan in critically ill patients with or at risk for acute renal failure: a meta-analysis of randomized clinical trials.Heart Lung Vessel. 2015;7(1):35-46. Heart Lung Vessel. 2015. PMID: 25861589 Free PMC article.
-
Intermittent inotrope therapy: evidence or belief?Clin Res Cardiol. 2015 Nov;104(11):998-9. doi: 10.1007/s00392-015-0903-7. Epub 2015 Aug 26. Clin Res Cardiol. 2015. PMID: 26306593 No abstract available.
-
Comparative efficacy of different drugs in acute heart failure with renal dysfunction: a systematic review and network meta-analysis.Front Cardiovasc Med. 2025 Jan 14;11:1444068. doi: 10.3389/fcvm.2024.1444068. eCollection 2024. Front Cardiovasc Med. 2025. PMID: 39877019 Free PMC article.
References
-
- Chatterjee K, Wolfe C L, De Marco T. Nonglycoside inotropes in congestive heart failure: are they beneficial or harmful? Cardiol Clin 19941263–72. - PubMed
-
- McBride B, White M. Levosimendan: implications for clinicians. J Clin Pharmacol 2003431071–1081. - PubMed
-
- Follath F, Cleland J G, Just H.et al Steering Committee and Investigators of the Levosimendan Infusion versus Dobutamine (LIDO) Study. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low‐output heart failure (the LIDO study): a randomised double‐blind trial, Lancet 2002360196–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous