Distinct mechanisms mediating methamphetamine-induced neuronal apoptosis and dopamine terminal damage share the neuropeptide substance p in the striatum of mice
- PMID: 17105911
- PMCID: PMC2892968
- DOI: 10.1196/annals.1369.013
Distinct mechanisms mediating methamphetamine-induced neuronal apoptosis and dopamine terminal damage share the neuropeptide substance p in the striatum of mice
Abstract
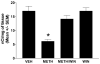
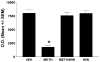
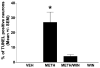
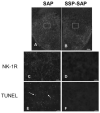
Methamphetamine (METH) is an addictive psychostimulant that induces damage to the dopamine terminals and the apoptosis of some neurons of the striatum. Our laboratory demonstrated using either a single bolus dose (30 mg/kg) or a binge (10 mg/kg 4x at 2-h intervals) of METH that pharmacological blockade of the substance P receptor (neurokinin-1) attenuates METH-induced damage to both the presynaptic dopamine terminals and the apoptosis of some neurons of the striatum. To determine the phenotype of striatal neuron ablated by METH, we combined TUNEL (Terminal Deoxyncleotidyl Transferase-Mediated dUTP Nick End Labeling) with immunofluorescence for selective markers of projection and interneurons. METH induces the loss of approximately 20% of the projection neurons. The cholinergic and gamma-aminobutyric acid (GABA)-parvalbumin interneurons sustain losses of 30% and 50%, respectively. The somatostatin/neuropeptide Y (NPY)/nitric oxide synthase (NOS) interneurons are not impacted by METH. To investigate the mechanism by which substance P mediates METH-induced damage in this part of the brain, we ablated the striatal interneurons that express the neurokinin-1 receptor (NK-1R) with the selective neurotoxin substance P-SAP. Ablation of the NK-1R-expressing interneurons prevented METH-induced apoptosis in the striatum but was without effect on depletion of dopamine terminal markers. We propose that substance P mediates the apoptosis of some striatal neurons via the intrastriatal activation of nitric oxide synthesis. In contrast, substance P may mediate damage of the dopamine terminals via an extrastriatal mechanism involving the substantia nigra and cortical glutamate release.
Figures

Similar articles
-
The neurokinin-1 receptor modulates the methamphetamine-induced striatal apoptosis and nitric oxide formation in mice.J Neurochem. 2009 Nov;111(3):656-68. doi: 10.1111/j.1471-4159.2009.06330.x. Epub 2009 Aug 13. J Neurochem. 2009. PMID: 19682209 Free PMC article.
-
Methamphetamine induces striatal neurokinin-1 receptor endocytosis primarily in somatostatin/NPY/NOS interneurons and the role of dopamine receptors in mice.Synapse. 2011 Apr;65(4):300-8. doi: 10.1002/syn.20848. Epub 2010 Sep 24. Synapse. 2011. PMID: 20730802 Free PMC article.
-
Methamphetamine-induced cell death: selective vulnerability in neuronal subpopulations of the striatum in mice.Neuroscience. 2006 Jun 30;140(2):607-22. doi: 10.1016/j.neuroscience.2006.02.055. Epub 2006 May 2. Neuroscience. 2006. PMID: 16650608 Free PMC article.
-
Synergism between methamphetamine and the neuropeptide substance P on the production of nitric oxide in the striatum of mice.Brain Res. 2011 Jan 19;1369:131-9. doi: 10.1016/j.brainres.2010.11.017. Epub 2010 Nov 11. Brain Res. 2011. PMID: 21075091 Free PMC article.
-
Substance P and cholecystokinin regulate neurochemical responses to cocaine and methamphetamine in the striatum.Life Sci. 2003 Jun 27;73(6):727-39. doi: 10.1016/s0024-3205(03)00393-x. Life Sci. 2003. PMID: 12801594 Review.
Cited by
-
Astrocytic clasmatodendrosis in the cerebral cortex of methamphetamine abusers.Forensic Sci Res. 2017 Jan 31;2(3):139-144. doi: 10.1080/20961790.2017.1280890. eCollection 2017. Forensic Sci Res. 2017. PMID: 30483632 Free PMC article.
-
Is there a role for nitric oxide in methamphetamine-induced dopamine terminal degeneration?Neurotox Res. 2014 Feb;25(2):153-60. doi: 10.1007/s12640-013-9415-2. Epub 2013 Aug 6. Neurotox Res. 2014. PMID: 23918001 Free PMC article. Review.
-
Evaluating the role of neuronal nitric oxide synthase-containing striatal interneurons in methamphetamine-induced dopamine neurotoxicity.Neurotox Res. 2013 Aug;24(2):288-97. doi: 10.1007/s12640-013-9391-6. Epub 2013 Apr 11. Neurotox Res. 2013. PMID: 23575992 Free PMC article.
-
The role of the neuropeptide somatostatin on methamphetamine and glutamate-induced neurotoxicity in the striatum of mice.Brain Res. 2013 May 13;1510:38-47. doi: 10.1016/j.brainres.2013.03.010. Epub 2013 Mar 19. Brain Res. 2013. PMID: 23524190 Free PMC article.
-
Glial cell modulators attenuate methamphetamine self-administration in the rat.Eur J Pharmacol. 2013 Feb 15;701(1-3):124-30. doi: 10.1016/j.ejphar.2013.01.016. Epub 2013 Jan 31. Eur J Pharmacol. 2013. PMID: 23375937 Free PMC article.
References
-
- Kogan FJ, Nichols WK, Gibb JW. Influence of methamphetamine on nigral and striatal tyrosine hydroxylase activity and on striatal dopamine levels. Eur. J. Pharmacol. 1976;36:363–371. - PubMed
-
- Seiden LS, Fischman MW, Schuster CR. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend. 1976;1:215–219. - PubMed
-
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J. Pharmacol. Exp. Ther. 1980;214:257–262. - PubMed
-
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. - PubMed
-
- Axt KJ, Molliver ME. Immunocytochemical evidence for methamphetamine-induced serotonergic axon loss in the rat brain. Synapse. 1991;9:302–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

