Expression patterns and action analysis of genes associated with blood coagulation responses during rat liver regeneration
- PMID: 17106934
- PMCID: PMC4087440
- DOI: 10.3748/wjg.v12.i42.6842
Expression patterns and action analysis of genes associated with blood coagulation responses during rat liver regeneration
Abstract
Aim: To study the blood coagulation response after partial hepatectomy (PH) at transcriptional level.
Methods: After PH of rats, the associated genes with blood coagulation were obtained through reference to the databases, and the gene expression changes in rat regenerating liver were analyzed by the Rat Genome 230 2.0 array.
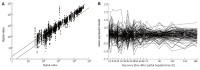
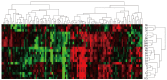
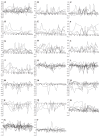
Results: It was found that 107 genes were associated with liver regeneration. The initially and totally expressing gene numbers occurring in initiation phase of liver regeneration (0.5-4 h after PH), G0/G1 transition (4-6 h after PH), cell proliferation (6-66 h after PH), cell differentiation and structure-function reconstruction (66-168 h after PH) were 44, 11, 58, 7 and 44, 33, 100, 71 respectively, showing that the associated genes were mainly triggered in the forepart and prophase, and worked at different phases. According to their expression similarity, these genes were classified into 5 groups: only up-, predominantly up-, only down-, predominantly down-, up- and down-regulation, involving 44, 8, 36, 13 and 6 genes, respectively, and the total times of their up- and down-regulation expression were 342 and 253, respectively, demonstrating that the number of the up-regulated genes was more than that of the down-regulated genes. Their time relevance was classified into 15 groups, showing that the cellular physiological and biochemical activities were staggered during liver regeneration. According to gene expression patterns, they were classified into 29 types, suggesting that their protein activities were diverse and complex during liver regeneration.
Conclusion: The blood coagulation response is enhanced mainly in the forepart, prophase and anaphase of liver regeneration, in which the response in the forepart, prophase of liver regeneration can prevent the bleeding caused by partial hepatectomy, whereas that in the anaphase contributes to the structure-function reorganization of regenerating liver. In the process, 107 genes associated with liver regeneration play an important role.
Figures
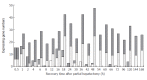
References
-
- Østerud B, Bjørklid E. Sources of tissue factor. Semin Thromb Hemost. 2006;32:11–23. - PubMed
-
- Machado FR, Silva E. Coagulation and sepsis. Endocr Metab Immune Disord Drug Targets. 2006;6:175–182. - PubMed
-
- Celikel R, McClintock RA, Roberts JR, Mendolicchio GL, Ware J, Varughese KI, Ruggeri ZM. Modulation of alpha-thrombin function by distinct interactions with platelet glycoprotein Ibalpha. Science. 2003;301:218–221. - PubMed
-
- Khrenov AV, Ananyeva NM, Saenko EL. Role of the B domain in proteolytic inactivation of activated coagulation factor VIII by activated protein C and activated factor X. Blood Coagul Fibrinolysis. 2006;17:379–388. - PubMed
-
- Wu SY, Wang ZY, Dong NZ, Zhang W, Bai X, Ruan CG. [A novel genetic defect in a Chinese family with inherited coagulation factor XIII deficiency] Zhonghua Xueyexue Zazhi. 2006;27:145–149. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

