Myotonia-related mutations in the distal C-terminus of ClC-1 and ClC-0 chloride channels affect the structure of a poly-proline helix
- PMID: 17107341
- PMCID: PMC1828897
- DOI: 10.1042/BJ20061230
Myotonia-related mutations in the distal C-terminus of ClC-1 and ClC-0 chloride channels affect the structure of a poly-proline helix
Abstract
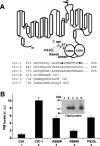
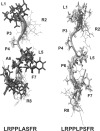
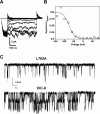
Myotonia is a state of hyperexcitability of skeletal-muscle fibres. Mutations in the ClC-1 Cl- channel cause recessive and dominant forms of this disease. Mutations have been described throughout the protein-coding region, including three sequence variations (A885P, R894X and P932L) in a distal C-terminal stretch of residues [CTD (C-terminal domain) region] that are not conserved between CLC proteins. We show that surface expression of these mutants is reduced in Xenopus oocytes compared with wild-type ClC-1. Functional, biochemical and NMR spectroscopy studies revealed that the CTD region encompasses a segment conserved in most voltage-dependent CLC channels that folds with a secondary structure containing a short type II poly-proline helix. We found that the myotonia-causing mutation A885P disturbs this structure by extending the poly-proline helix. We hypothesize that this structural modification results in the observed alteration of the common gate that acts on both pores of the channel. We provide the first experimental investigation of structural changes resulting from myotonia-causing mutations.
Figures




Similar articles
-
Characterization of two new dominant ClC-1 channel mutations associated with myotonia.Muscle Nerve. 2003 Dec;28(6):722-32. doi: 10.1002/mus.10501. Muscle Nerve. 2003. PMID: 14639587 Clinical Trial.
-
ClC-1 mutations in myotonia congenita patients: insights into molecular gating mechanisms and genotype-phenotype correlation.J Physiol. 2015 Sep 15;593(18):4181-99. doi: 10.1113/JP270358. Epub 2015 Jul 14. J Physiol. 2015. PMID: 26096614 Free PMC article.
-
The muscle chloride channel ClC-1 has a double-barreled appearance that is differentially affected in dominant and recessive myotonia.J Gen Physiol. 1999 Mar;113(3):457-68. doi: 10.1085/jgp.113.3.457. J Gen Physiol. 1999. PMID: 10051520 Free PMC article.
-
Myotonia caused by mutations in the muscle chloride channel gene CLCN1.Hum Mutat. 2002 Apr;19(4):423-34. doi: 10.1002/humu.10063. Hum Mutat. 2002. PMID: 11933197 Review.
-
Molecular mechanisms of ion conduction in ClC-type chloride channels: lessons from disease-causing mutations.Kidney Int. 2000 Mar;57(3):780-6. doi: 10.1046/j.1523-1755.2000.00915.x. Kidney Int. 2000. PMID: 10720929 Review.
Cited by
-
Dominantly inherited myotonia congenita resulting from a mutation that increases open probability of the muscle chloride channel CLC-1.Neuromolecular Med. 2012 Dec;14(4):328-37. doi: 10.1007/s12017-012-8190-1. Epub 2012 Jul 12. Neuromolecular Med. 2012. PMID: 22790975 Free PMC article.
-
Regulation of CLC-1 chloride channel biosynthesis by FKBP8 and Hsp90β.Sci Rep. 2016 Sep 1;6:32444. doi: 10.1038/srep32444. Sci Rep. 2016. PMID: 27580824 Free PMC article.
-
Proton-dependent inhibition, inverted voltage activation, and slow gating of CLC-0 Chloride Channel.PLoS One. 2020 Dec 23;15(12):e0240704. doi: 10.1371/journal.pone.0240704. eCollection 2020. PLoS One. 2020. PMID: 33362212 Free PMC article.
-
Novel brain expression of ClC-1 chloride channels and enrichment of CLCN1 variants in epilepsy.Neurology. 2013 Mar 19;80(12):1078-85. doi: 10.1212/WNL.0b013e31828868e7. Epub 2013 Feb 13. Neurology. 2013. PMID: 23408874 Free PMC article.
-
FKBP8 Enhances Protein Stability of the CLC-1 Chloride Channel at the Plasma Membrane.Int J Mol Sci. 2018 Nov 28;19(12):3783. doi: 10.3390/ijms19123783. Int J Mol Sci. 2018. PMID: 30487393 Free PMC article.
References
-
- Koch M. C., Steinmeyer K., Lorenz C., Ricker K., Wolf F., Otto M., Zoll B., Lehmann-Horn F., Grzeschik K. H., Jentsch T. J. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992;257:797–800. - PubMed
-
- George A. L., Jr, Crackower M. A., Abdalla J. A., Hudson A. J., Ebers G. C. Molecular basis of Thomsen's disease (autosomal dominant myotonia congenita) Nat. Genet. 1993;3:305–310. - PubMed
-
- Jentsch T. J., Lorenz C., Pusch M., Steinmeyer K. Myotonias due to CLC-1 chloride channel mutations. Soc. Gen. Physiol. Ser. 1995;50:149–159. - PubMed
-
- Pusch M. Myotonia caused by mutations in the muscle chloride channel gene CLCN1. Hum. Mutat. 2002;19:423–434. - PubMed
-
- Jentsch T. J., Poet M., Fuhrmann J. C., Zdebik A. A. Physiological functions of CLC Cl− channels gleaned from human genetic disease and mouse models. Annu. Rev. Physiol. 2005;67:779–807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

