A severe acute respiratory syndrome coronavirus that lacks the E gene is attenuated in vitro and in vivo
- PMID: 17108030
- PMCID: PMC1797558
- DOI: 10.1128/JVI.01467-06
A severe acute respiratory syndrome coronavirus that lacks the E gene is attenuated in vitro and in vivo
Abstract
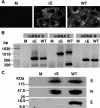
A deletion mutant of severe acute respiratory syndrome coronavirus (SARS-CoV) has been engineered by deleting the structural E gene in an infectious cDNA clone that was constructed as a bacterial artificial chromosome (BAC). The recombinant virus lacking the E gene (rSARS-CoV-DeltaE) was rescued in Vero E6 cells. The recovered deletion mutant grew in Vero E6, Huh-7, and CaCo-2 cells to titers 20-, 200-, and 200-fold lower than the recombinant wild-type virus, respectively, indicating that although the E protein has an effect on growth, it is not essential for virus replication. No differences in virion stability under a wide range of pH and temperature were detected between the deletion mutant and recombinant wild-type viruses. Although both viruses showed the same morphology by electron microscopy, the process of morphogenesis seemed to be less efficient with the defective virus than with the recombinant wild-type one. The rSARS-CoV-DeltaE virus replicated to titers 100- to 1,000-fold lower than the recombinant wild-type virus in the upper and lower respiratory tract of hamsters, and the lower viral load was accompanied by less inflammation in the lungs of hamsters infected with rSARS-CoV-DeltaE virus than with the recombinant wild-type virus. Therefore, the SARS-CoV that lacks the E gene is attenuated in hamsters, might be a safer research tool, and may be a good candidate for the development of a live attenuated SARS-CoV vaccine.
Figures




References
-
- Almazán, F., M. L. DeDiego, C. Galan, D. Escors, E. Alvarez, J. Ortego, I. Sola, S. Zuñiga, S. Alonso, J. L. Moreno, A. Nogales, C. Capiscol, and L. Enjuanes. 2006. Construction of a SARS-CoV infectious cDNA clone and a replicon to study coronavirus RNA synthesis. J. Virol. 80:10900-10906. - PMC - PubMed
-
- Chu, C. M., L. L. Poon, V. C. Cheng, K. S. Chan, I. F. Hung, M. M. Wong, K. H. Chan, W. S. Leung, B. S. Tang, V. L. Chan, W. L. Ng, T. C. Sim, P. W. Ng, K. I. Law, D. M. Tse, J. S. Peiris, and K. Y. Yuen. 2004. Initial viral load and the outcomes of SARS. Can. Med. Assoc. J. 171:1349-1352. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

