Human immunodeficiency virus type 1 matrix protein assembles on membranes as a hexamer
- PMID: 17108052
- PMCID: PMC1797500
- DOI: 10.1128/JVI.02122-06
Human immunodeficiency virus type 1 matrix protein assembles on membranes as a hexamer
Abstract
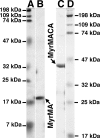
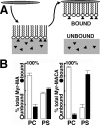
The membrane-binding matrix (MA) domain of the human immunodeficiency virus type 1 (HIV-1) structural precursor Gag (PrGag) protein oligomerizes in solution as a trimer and crystallizes in three dimensions as a trimer unit. A number of models have been proposed to explain how MA trimers might align with respect to PrGag capsid (CA) N-terminal domains (NTDs), which assemble hexagonal lattices. We have examined the binding of naturally myristoylated HIV-1 matrix (MyrMA) and matrix plus capsid (MyrMACA) proteins on membranes in vitro. Unexpectedly, MyrMA and MyrMACA proteins both assembled hexagonal cage lattices on phosphatidylserine-cholesterol membranes. Membrane-bound MyrMA proteins did not organize into trimer units but, rather, organized into hexamer rings. Our results yield a model in which MA domains stack directly above NTD hexamers in immature particles, and they have implications for HIV assembly and interactions between MA and the viral membrane glycoproteins.
Figures



References
-
- Amos, L., R. Henderson, and P. Unwin. 1982. Three-dimensional structure determination by electron microscopy of two-dimensional crystals. Prog. Biophys. Mol. Biol. 39:183-231. - PubMed
-
- Barklis, E., J. McDermott, S. Wilkens, S. Fuller, and D. Thompson. 1998. Organization of HIV-1 capsid proteins on a lipid monolayer. J. Biol. Chem. 273:7177-7180. - PubMed
-
- Bouamr, F., S. Scarlata, and C. Carter. 2003. Role of myristoylation in HIV-1 Gag assembly. Biochemistry 42:6408-6417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

