Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns
- PMID: 17108163
- PMCID: PMC6674856
- DOI: 10.1523/JNEUROSCI.2044-06.2006
Cranial visceral afferent pathways through the nucleus of the solitary tract to caudal ventrolateral medulla or paraventricular hypothalamus: target-specific synaptic reliability and convergence patterns
Abstract
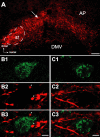
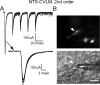
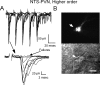
Cranial visceral afferents activate central pathways that mediate systemic homeostatic processes. Afferent information arrives in the brainstem nucleus of the solitary tract (NTS) and is relayed to other CNS sites for integration into autonomic responses and complex behaviors. Little is known about the organization or nature of processing within NTS. We injected fluorescent retrograde tracers into two nuclei to identify neurons that project to sites involved in autonomic regulation: the caudal ventrolateral medulla (CVLM) or paraventricular nucleus of the hypothalamus (PVN). We found distinct differences in synaptic connections and performance in the afferent path through NTS to these neurons. Anatomical studies using confocal and electron microscopy found prominent, primary afferent synapses directly on somata and dendrites of CVLM-projecting NTS neurons identifying them as second-order neurons. In brainstem slices, afferent activation evoked large, constant latency EPSCs in CVLM-projecting NTS neurons that were consistent with the precise timing and rare failures of monosynaptic contacts on second-order neurons. In contrast, most PVN-projecting NTS neurons lacked direct afferent input and responded to afferent stimuli with highly variable, intermittently failing synaptic responses, indicating polysynaptic pathways to higher-order neurons. The afferent-evoked EPSCs in most PVN-projecting NTS neurons were smaller and unreliable but also often included multiple, convergent polysynaptic responses not observed in CVLM-projecting neurons. A few PVN-projecting NTS neurons had monosynaptic EPSC characteristics. Together, we found that cranial visceral afferent pathways are structured distinctly within NTS depending on the projection target. Such, intra-NTS pathway architecture will substantially impact performance of autonomic or neuroendocrine reflex arcs.
Figures






References
-
- Aicher SA, Kurucz OS, Reis DJ, Milner TA. Nucleus tractus solitarius efferent terminals synapse on neurons in the caudal ventrolateral medulla that project to the rostral ventrolateral medulla. Brain Res. 1995;693:51–63. - PubMed
-
- Aicher SA, Saravay RH, Cravo S, Jeske I, Morrison SF, Reis DJ, Milner TA. Monosynaptic projections from the nucleus tractus solitarii to C1 adrenergic neurons in the rostral ventrolateral medulla: comparison with input from the caudal ventrolateral medulla. J Comp Neurol. 1996;373:62–75. - PubMed
-
- Aicher SA, Sharma S, Cheng PY, Pickel VM. The N-methyl-d-aspartate (NMDA) receptor is postsynaptic to substance P-containing axon terminals in the rat superficial dorsal horn. Brain Res. 1997;772:71–81. - PubMed
-
- Aicher SA, Sharma S, Pickel VM. N-methyl-d-aspartate receptors are present in vagal afferents and their dendritic targets in the nucleus tractus solitarius. Neuroscience. 1999;91:119–132. - PubMed
-
- Aicher SA, Milner TA, Pickel VM, Reis DJ. Anatomical substrates for baroreflex sympathoinhibition in the rat. Brain Res Bull. 2000;51:107–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
