Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis
- PMID: 17108165
- PMCID: PMC6674868
- DOI: 10.1523/JNEUROSCI.3821-06.2006
Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis
Abstract
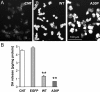
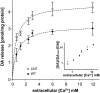
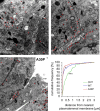
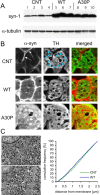
Alpha-synuclein (alpha-syn), a protein implicated in Parkinson's disease pathogenesis, is a presynaptic protein suggested to regulate transmitter release. We explored how alpha-syn overexpression in PC12 and chromaffin cells, which exhibit low endogenous alpha-syn levels relative to neurons, affects catecholamine release. Overexpression of wild-type or A30P mutant alpha-syn in PC12 cell lines inhibited evoked catecholamine release without altering calcium threshold or cooperativity of release. Electron micrographs revealed that vesicular pools were not reduced but that, on the contrary, a marked accumulation of morphologically "docked" vesicles was apparent in the alpha-syn-overexpressing lines. We used amperometric recordings from chromaffin cells derived from mice that overexpress A30P or wild-type (WT) alpha-syn, as well as chromaffin cells from control and alpha-syn null mice, to determine whether the filling of vesicles with the transmitter was altered. The quantal size and shape characteristics of amperometric events were identical for all mouse lines, suggesting that overexpression of WT or mutant alpha-syn did not affect vesicular transmitter accumulation or the kinetics of vesicle fusion. The frequency and number of exocytotic events per stimulus, however, was lower for both WT and A30P alpha-syn-overexpressing cells. The alpha-syn-overexpressing cells exhibited reduced depression of evoked release in response to repeated stimuli, consistent with a smaller population of readily releasable vesicles. We conclude that alpha-syn overexpression inhibits a vesicle "priming" step, after secretory vesicle trafficking to "docking" sites but before calcium-dependent vesicle membrane fusion.
Figures
Comment in
-
Prime time for alpha-synuclein.J Neurosci. 2007 Mar 7;27(10):2433-4. doi: 10.1523/JNEUROSCI.0094-07.2007. J Neurosci. 2007. PMID: 17344380 Free PMC article. No abstract available.
References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
-
- Bayer TA, Jakala P, Hartmann T, Egensperger R, Buslei R, Falkai P, Beyreuther K. Neural expression profile of alpha-synuclein in developing human cortex. NeuroReport. 1999;10:2799–2803. - PubMed
-
- Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J Neurosci. 2002;22:8797–8807. - PMC - PubMed
-
- Chandra S, Gallardo G, Fernandez-Chacon R, Schluter OM, Sudhof TC. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
