Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid beta, amyloid beta40, and amyloid beta42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice
- PMID: 17108166
- PMCID: PMC6674861
- DOI: 10.1523/JNEUROSCI.2795-06.2006
Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid beta, amyloid beta40, and amyloid beta42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice
Erratum in
- J Neurosci. 2006 Dec 6;26(49):preceding 12847
Abstract
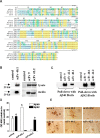
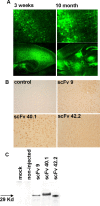
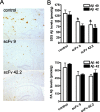
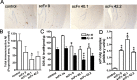
Accumulation of amyloid beta protein (Abeta) aggregates is hypothesized to trigger a pathological cascade that causes Alzheimer's disease (AD). Active or passive immunizations targeting Abeta are therefore of great interest as potential therapeutic strategies. We have evaluated the use of recombinant anti-Abeta single-chain variable fragments (scFvs) as a potentially safer form of anti-Abeta immunotherapy. We have generated and characterized three anti-Abeta scFvs that recognize Abeta 1-16, Abeta x-40, or Abeta x-42. To achieve widespread brain delivery, constructs expressing these anti-Abeta scFvs were packaged into adeno-associated virus (AAV) vectors and injected into the ventricles of postnatal day 0 (P0) amyloid precursor protein CRND8-transgenic mice. Intracranial delivery of AAV to neonatal mice resulted in widespread neuronal delivery. In situ expression of each of the anti-Abeta scFvs after intracerebroventricular AAV serotype 1 delivery to P0 pups decreased Abeta deposition by 25-50%. These data suggest that intracranial anti-Abeta scFv expression is an effective strategy to attenuate amyloid deposition. As opposed to transgenic approaches, these studies also establish a "somatic brain transgenic" paradigm to rapidly and cost-effectively evaluate potential modifiers of AD-like pathology in AD mouse models.
Figures
Similar articles
-
Anti-Abeta single-chain antibody delivery via adeno-associated virus for treatment of Alzheimer's disease.Neurobiol Dis. 2006 Sep;23(3):502-11. doi: 10.1016/j.nbd.2006.04.012. Neurobiol Dis. 2006. PMID: 16766200 Free PMC article.
-
A novel recombinant adeno-associated virus vaccine reduces behavioral impairment and beta-amyloid plaques in a mouse model of Alzheimer's disease.Neurobiol Dis. 2003 Dec;14(3):365-79. doi: 10.1016/j.nbd.2003.07.005. Neurobiol Dis. 2003. PMID: 14678754
-
Improved Brain Expression of Anti-Amyloid β scFv by Complexation of mRNA Including a Secretion Sequence with PEG-based Block Catiomer.Curr Alzheimer Res. 2017;14(3):295-302. doi: 10.2174/1567205013666161108110031. Curr Alzheimer Res. 2017. PMID: 27829339
-
Intracerebroventricular passive immunization in transgenic mouse models of Alzheimer's disease.Expert Rev Vaccines. 2004 Dec;3(6):717-25. doi: 10.1586/14760584.3.6.717. Expert Rev Vaccines. 2004. PMID: 15606357 Review.
-
Amyloid-beta immunotherapy for Alzheimer's disease.CNS Neurol Disord Drug Targets. 2010 Apr;9(2):197-206. doi: 10.2174/187152710791012017. CNS Neurol Disord Drug Targets. 2010. PMID: 20205640 Free PMC article. Review.
Cited by
-
Intracerebroventricular amyloid-beta antibodies reduce cerebral amyloid angiopathy and associated micro-hemorrhages in aged Tg2576 mice.Proc Natl Acad Sci U S A. 2009 Mar 17;106(11):4501-6. doi: 10.1073/pnas.0813404106. Epub 2009 Feb 25. Proc Natl Acad Sci U S A. 2009. PMID: 19246392 Free PMC article.
-
Open questions for Alzheimer's disease immunotherapy.Alzheimers Res Ther. 2014 Jan 7;6(1):3. doi: 10.1186/alzrt233. eCollection 2014. Alzheimers Res Ther. 2014. PMID: 24393284 Free PMC article. Review.
-
Tailored transgene expression to specific cell types in the central nervous system after peripheral injection with AAV9.Mol Ther Methods Clin Dev. 2016 Dec 7;3:16081. doi: 10.1038/mtm.2016.81. eCollection 2016. Mol Ther Methods Clin Dev. 2016. PMID: 27933308 Free PMC article.
-
Adeno-associated virus vector delivery to the brain: Technology advancements and clinical applications.Adv Drug Deliv Rev. 2024 Aug;211:115363. doi: 10.1016/j.addr.2024.115363. Epub 2024 Jun 19. Adv Drug Deliv Rev. 2024. PMID: 38906479 Free PMC article. Review.
-
BRI2 (ITM2b) inhibits Abeta deposition in vivo.J Neurosci. 2008 Jun 4;28(23):6030-6. doi: 10.1523/JNEUROSCI.0891-08.2008. J Neurosci. 2008. PMID: 18524908 Free PMC article.
References
-
- Banks WA, Pagliari P, Nakaoke R, Morley JE. Effects of a behaviorally active antibody on the brain uptake and clearance of amyloid beta proteins. Peptides. 2005;26:287–294. - PubMed
-
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. - PubMed
-
- Bennett DA, Holtzman DM. Immunization therapy for Alzheimer disease? Neurology. 2005;64:10–12. - PubMed
-
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer's disease-linked presenilin 1 variants elevate Abeta1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
