Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility
- PMID: 17108170
- PMCID: PMC6674881
- DOI: 10.1523/JNEUROSCI.3171-06.2006
Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility
Abstract
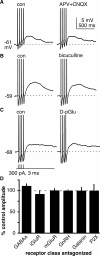
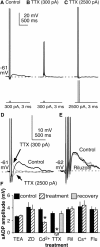
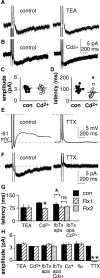
The brain controls fertility through release of gonadotropin-releasing hormone (GnRH), but the mechanisms underlying action potential patterning and GnRH release are not understood. We investigated whether GnRH neurons exhibit afterdepolarizing potentials (ADPs) and whether these are modified by reproductive state. Whole-cell current-clamp recordings of GnRH neurons in brain slices from ovariectomized mice revealed a slow ADP (sADP) after action potentials generated by brief current injection. Generating two or four spikes enhanced sADP amplitude and duration. sADP amplitude was not affected by blocking selected neurotransmitter/neuromodulator receptors, delayed-rectifier potassium channels, calcium-dependent cation channels, or hyperpolarization-activated cation channels but was halved by the calcium channel blocker cadmium and abolished by tetrodotoxin. Cadmium also reduced peak latency. Intrinsic mechanisms underlying the sADP were investigated using voltage-clamp protocols simulating action potential waveforms. A single action potential produced an inward current, which increased after double and quadruple stimulation. Cadmium did not affect current amplitude but reduced peak latency. Pretreatment with blockers of calcium-activated potassium currents (I(KCa)) reproduced this shift and blocked subsequent cadmium-induced changes, suggesting cadmium changes latency indirectly by blocking I(KCa). Tetrodotoxin abolished the inward current, suggesting that it is carried by sodium. In contrast, I(KCa) blockers increased the inward current, indicating that I(KCa) may oppose generation of the sADP. Strong sADPs were suprathreshold, generating repetitive spontaneous firing. I(ADP), sADP, and excitability were enhanced by in vivo estradiol, which triggers a preovulatory surge of GnRH release. Physiological feedback modification of this inward current and resulting sADP may modulate action potential firing and subsequent GnRH release.
Figures





Similar articles
-
Estradiol attenuates multiple tetrodotoxin-sensitive sodium currents in isolated gonadotropin-releasing hormone neurons.Brain Res. 2010 Jul 23;1345:137-45. doi: 10.1016/j.brainres.2010.05.031. Epub 2010 May 16. Brain Res. 2010. PMID: 20580637 Free PMC article.
-
Excitability and Burst Generation of AVPV Kisspeptin Neurons Are Regulated by the Estrous Cycle Via Multiple Conductances Modulated by Estradiol Action.eNeuro. 2016 Jun 7;3(3):ENEURO.0094-16.2016. doi: 10.1523/ENEURO.0094-16.2016. eCollection 2016 May-Jun. eNeuro. 2016. PMID: 27280155 Free PMC article.
-
Heterogeneity in the basic membrane properties of postnatal gonadotropin-releasing hormone neurons in the mouse.J Neurosci. 2001 Feb 1;21(3):1067-75. doi: 10.1523/JNEUROSCI.21-03-01067.2001. J Neurosci. 2001. PMID: 11157093 Free PMC article.
-
Autocrine regulation of calcium influx and gonadotropin-releasing hormone secretion in hypothalamic neurons.Biochem Cell Biol. 2000;78(3):359-70. Biochem Cell Biol. 2000. PMID: 10949086 Review.
-
Kisspeptin and Gonadotropin-Releasing Hormone Neuronal Excitability: Molecular Mechanisms Driven by 17β-Estradiol.Neuroendocrinology. 2015;102(3):184-93. doi: 10.1159/000370311. Epub 2014 Dec 8. Neuroendocrinology. 2015. PMID: 25612870 Free PMC article. Review.
Cited by
-
Critical roles for fast synaptic transmission in mediating estradiol negative and positive feedback in the neural control of ovulation.Endocrinology. 2008 Nov;149(11):5500-8. doi: 10.1210/en.2008-0453. Epub 2008 Jul 10. Endocrinology. 2008. PMID: 18617615 Free PMC article.
-
Proestrus Differentially Regulates Expression of Ion Channel and Calcium Homeostasis Genes in GnRH Neurons of Mice.Front Mol Neurosci. 2019 May 31;12:137. doi: 10.3389/fnmol.2019.00137. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31213979 Free PMC article.
-
Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons.J Neurosci. 2010 Mar 17;30(11):3912-23. doi: 10.1523/JNEUROSCI.6256-09.2010. J Neurosci. 2010. PMID: 20237262 Free PMC article.
-
Changes in Both Neuron Intrinsic Properties and Neurotransmission Are Needed to Drive the Increase in GnRH Neuron Firing Rate during Estradiol-Positive Feedback.J Neurosci. 2019 Mar 13;39(11):2091-2101. doi: 10.1523/JNEUROSCI.2880-18.2019. Epub 2019 Jan 17. J Neurosci. 2019. PMID: 30655354 Free PMC article.
-
Neurobiological mechanisms underlying oestradiol negative and positive feedback regulation of gonadotrophin-releasing hormone neurones.J Neuroendocrinol. 2009 Mar;21(4):327-33. doi: 10.1111/j.1365-2826.2009.01826.x. J Neuroendocrinol. 2009. PMID: 19207821 Free PMC article. Review.
References
-
- Adams MM, Flagg RA, Gore AC. Perinatal changes in hypothalamic N-methyl-d-aspartate receptors and their relationship to gonadotropin-releasing hormone neurons. Endocrinology. 1999;140:2288–2296. - PubMed
-
- Agrawal N, Hamam BN, Magistretti J, Alonso A, Ragsdale DS. Persistent sodium channel activity mediates subthreshold membrane potential oscillations and low-threshold spikes in rat entorhinal cortex layer V neurons. Neuroscience. 2001;102:53–64. - PubMed
-
- Alonso A, Llinas RR. Subtreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature. 1989;342:175–177. - PubMed
-
- Andrew RD, Dudek FE. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983;221:1050–1052. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
