Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain
- PMID: 17108182
- PMCID: PMC6674883
- DOI: 10.1523/JNEUROSCI.3047-06.2006
Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain
Abstract
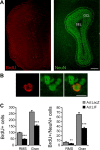
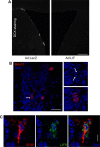
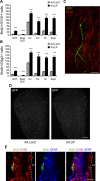
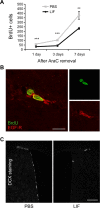
Although neural stem cells (NSCs) persist in various areas of the adult brain, their contribution to brain repair after injury is very limited. Treatment with exogenous growth factors can mitigate this limitation, suggesting that the brain environment is normally deficient in permissive cues and that it may be possible to stimulate the latent regenerative potential of endogenous progenitors with appropriate signals. We analyzed the effects of overexpressing the cytokine leukemia inhibitory factor (LIF) on adult neurogenesis in the normal brain. We found that LIF reduces neurogenesis in the olfactory bulb and subventricular zone by acting directly on NSCs. LIF appears to promote NSC self-renewal, preventing the emergence of more differentiated cell types. This ultimately leads to an expansion of the NSC pool. Our results have implications for the development of therapeutic strategies for brain repair and suggest that LIF may be useful, in combination with other factors, in promoting regeneration in the adult brain.
Figures

References
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
-
- Banner LR, Moayeri NN, Patterson PH. Leukemia inhibitory factor is expressed in astrocytes following cortical brain injury. Exp Neurol. 1997;147:1–9. - PubMed
-
- Barnabe-Heider F, Wasylnka JA, Fernandes KJ, Porsche C, Sendtner M, Kaplan DR, Miller FD. Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron. 2005;48:253–265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
