Characterization of AtNUC-L1 reveals a central role of nucleolin in nucleolus organization and silencing of AtNUC-L2 gene in Arabidopsis
- PMID: 17108323
- PMCID: PMC1783796
- DOI: 10.1091/mbc.e06-08-0751
Characterization of AtNUC-L1 reveals a central role of nucleolin in nucleolus organization and silencing of AtNUC-L2 gene in Arabidopsis
Abstract
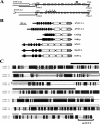
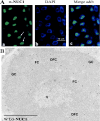
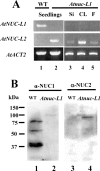
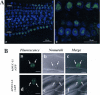
Nucleolin is one of the most abundant protein in the nucleolus and is a multifunctional protein involved in different steps of ribosome biogenesis. In contrast to animals and yeast, the genome of the model plant Arabidopsis thaliana encodes two nucleolin-like proteins, AtNUC-L1 and AtNUC-L2. However, only the AtNUC-L1 gene is ubiquitously expressed in normal growth conditions. Disruption of this AtNUC-L1 gene leads to severe plant growth and development defects. AtNUC-L1 is localized in the nucleolus, mainly in the dense fibrillar component. Absence of this protein in Atnuc-L1 plants induces nucleolar disorganization, nucleolus organizer region decondensation, and affects the accumulation levels of pre-rRNA precursors. Remarkably, in Atnuc-L1 plants the AtNUC-L2 gene is activated, suggesting that AtNUC-L2 might rescue, at least partially, the loss of AtNUC-L1. This work is the first description of a higher eukaryotic organism with a disrupted nucleolin-like gene and defines a new role for nucleolin in nucleolus structure and rDNA chromatin organization.
Figures



Similar articles
-
Sugar-inducible expression of the nucleolin-1 gene of Arabidopsis thaliana and its role in ribosome synthesis, growth and development.Plant J. 2007 Mar;49(6):1053-63. doi: 10.1111/j.1365-313X.2006.03016.x. Epub 2007 Feb 7. Plant J. 2007. PMID: 17286797
-
Nucleolin is required for DNA methylation state and the expression of rRNA gene variants in Arabidopsis thaliana.PLoS Genet. 2010 Nov 24;6(11):e1001225. doi: 10.1371/journal.pgen.1001225. PLoS Genet. 2010. PMID: 21124873 Free PMC article.
-
Nucleolar localization of aprataxin is dependent on interaction with nucleolin and on active ribosomal DNA transcription.Hum Mol Genet. 2006 Jul 15;15(14):2239-49. doi: 10.1093/hmg/ddl149. Epub 2006 Jun 15. Hum Mol Genet. 2006. PMID: 16777843
-
Plant nucleolar DNA: Green light shed on the role of Nucleolin in genome organization.Nucleus. 2017 Jan 2;8(1):11-16. doi: 10.1080/19491034.2016.1236167. Epub 2016 Sep 20. Nucleus. 2017. PMID: 27644794 Free PMC article. Review.
-
Structure and functions of nucleolin.J Cell Sci. 1999 Mar;112 ( Pt 6):761-72. doi: 10.1242/jcs.112.6.761. J Cell Sci. 1999. PMID: 10036227 Review.
Cited by
-
Nuclear dynamics: Formation of bodies and trafficking in plant nuclei.Front Plant Sci. 2022 Aug 23;13:984163. doi: 10.3389/fpls.2022.984163. eCollection 2022. Front Plant Sci. 2022. PMID: 36082296 Free PMC article. Review.
-
Arabidopsis RIBOSOMAL RNA PROCESSING7 Is Required for 18S rRNA Maturation.Plant Cell. 2018 Nov;30(11):2855-2872. doi: 10.1105/tpc.18.00245. Epub 2018 Oct 25. Plant Cell. 2018. PMID: 30361235 Free PMC article.
-
Light and gravity signals synergize in modulating plant development.Front Plant Sci. 2014 Oct 28;5:563. doi: 10.3389/fpls.2014.00563. eCollection 2014. Front Plant Sci. 2014. PMID: 25389428 Free PMC article. Review.
-
Nucleolus-tethering system (NoTS) reveals that assembly of photobodies follows a self-organization model.Mol Biol Cell. 2014 Apr;25(8):1366-73. doi: 10.1091/mbc.E13-09-0527. Epub 2014 Feb 19. Mol Biol Cell. 2014. PMID: 24554768 Free PMC article.
-
Nucleolin: The most abundant multifunctional phosphoprotein of nucleolus.Commun Integr Biol. 2011 May;4(3):267-75. doi: 10.4161/cib.4.3.14884. Commun Integr Biol. 2011. PMID: 21980556 Free PMC article.
References
-
- Bouvet P., Diaz J. J., Kindbeiter K., Madjar J. J., Amalric F. Nucleolin interacts with several ribosomal proteins through its RGG domain. J. Biol. Chem. 1998;273:19025–19029. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

