Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process
- PMID: 17108328
- PMCID: PMC1783799
- DOI: 10.1091/mbc.e06-06-0487
Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process
Abstract
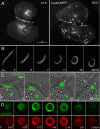
Beyond its well-documented role in vesicle endocytosis, clathrin has also been implicated in the internalization of large particles such as viruses, pathogenic bacteria, and even latex beads. We have discovered an additional clathrin-dependent endocytic process that results in the internalization of large, double-membrane vesicles at lateral membranes of cells that are coupled by gap junctions (GJs). GJ channels bridge apposing cell membranes to mediate the direct transfer of electrical currents and signaling molecules from cell to cell. Here, we report that entire GJ plaques, clusters of GJ channels, can be internalized to form large, double-membrane vesicles previously termed annular gap junctions (AGJs). These internalized AGJ vesicles subdivide into smaller vesicles that are degraded by endo/lysosomal pathways. Mechanistic analyses revealed that clathrin-dependent endocytosis machinery-components, including clathrin itself, the alternative clathrin-adaptor Dab2, dynamin, myosin-VI, and actin are involved in the internalization, inward movement, and degradation of these large, intercellular double-membrane vesicles. These findings contribute to the understanding of clathrin's numerous emerging functions.
Figures





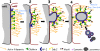
References
-
- Beardslee M. A., Laing J. G., Beyer E. C., Saffitz J. E. Rapid turnover of connexin43 in the adult rat heart. Circ. Res. 1998;83:629–635. - PubMed
-
- Berthoud V. M., Minogue P. J., Laing J. G., Beyer E. C. Pathways for degradation of connexins and gap junctions. Cardiovasc. Res. 2004;62:256–267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

