Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector
- PMID: 17109335
- PMCID: PMC2428071
- DOI: 10.1086/509258
Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector
Erratum in
- J Infect Dis. 2009 Oct 15;200(8):1352-3
Abstract
Background: The development of an effective human immunodeficiency virus (HIV) vaccine is a high global priority. Here, we report the safety, tolerability, and immunogenicity of a replication-defective recombinant adenovirus serotype 5 (rAd5) vector HIV-1 candidate vaccine.
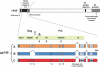
Methods: The vaccine is a mixture of 4 rAd5 vectors that express HIV-1 subtype B Gag-Pol fusion protein and envelope (Env) from subtypes A, B, and C. Healthy, uninfected adults were randomized to receive 1 intramuscular injection of placebo (n=6) or vaccine at dose levels of 10(9) (n=10), 10(10) (n=10), or 10(11) (n=10) particle units and were followed for 24 weeks to assess immunogenicity and safety.
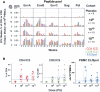
Results: The vaccine was well tolerated but was associated with more reactogenicity at the highest dose. At week 4, vaccine antigen-specific T cell responses were detected in 28 (93.3%) and 18 (60%) of 30 vaccine recipients for CD4(+) and CD8(+) T cells, respectively, by intracellular cytokine staining assay and in 22 (73%) of 30 vaccine recipients by enzyme-linked immunospot assay. Env-specific antibody responses were detected in 15 (50%) of 30 vaccine recipients by enzyme-linked immunosorbant assay and in 28 (93.3%) of 30 vaccine recipients by immunoprecipitation followed by Western blotting. No neutralizing antibody was detected.
Conclusions: A single injection induced HIV-1 antigen-specific CD4(+) T cell, CD8(+) T cell, and antibody responses in the majority of vaccine recipients. This multiclade rAd5 HIV-1 vaccine is now being evaluated in combination with a multiclade HIV-1 DNA plasmid vaccine.
Trial registration: ClinicalTrials.gov NCT00083330.
Figures



Comment in
-
Phase 1 clinical trials of the National Institutes of Health Vaccine Research Center HIV/AIDS candidate vaccines.J Infect Dis. 2006 Dec 15;194(12):1625-7. doi: 10.1086/509263. Epub 2006 Nov 8. J Infect Dis. 2006. PMID: 17109331 No abstract available.
References
-
- UNAIDS/WHO . AIDS epidemic update: December 2005. UNAIDS; Geneva: 2005.
-
- Barouch DH, Nabel GJ. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum Gene Ther. 2005;16:149–56. - PubMed
-
- Clark KR, Johnson PR. Gene delivery of vaccines for infectious disease. Curr Opin Mol Ther. 2001;3:375–84. - PubMed
-
- Shiver JW, Emini EA. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Ann Rev Med. 2004;55:355–72. - PubMed
-
- Shiver JW, Fu TM, Chen L, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

