Importin alpha1 is involved in the nuclear localization of Zac1 and the induction of p21WAF1/CIP1 by Zac1
- PMID: 17109628
- PMCID: PMC1798434
- DOI: 10.1042/BJ20061295
Importin alpha1 is involved in the nuclear localization of Zac1 and the induction of p21WAF1/CIP1 by Zac1
Abstract
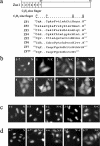
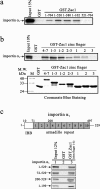
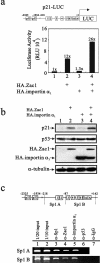
Zac1, a novel seven-zinc-finger transcription factor, preferentially binds GC-rich DNA elements and has intrinsic transactivation activity. To date, the NLS (nuclear localization signal) of Zac1 has not been empirically determined. We generated a series of EGFP (enhanced green fluorescence protein)-tagged deletion mutants of Zac1 and examined their subcellular localization, from which we defined two NLSs within the DNA-binding (or zinc-finger) domain. Fusion proteins consisting of the two EGFP-tagged zinc-finger clusters (zinc finger motifs 1-3 and 4-7) were located exclusively in the nucleus, demonstrating that each of the zinc-finger clusters is sufficient for nuclear localization. Physical interactions between these two zinc-finger clusters and importin alpha1 were demonstrated using an in vitro glutathione S-transferase pull-down assay. Finally, our results indicate that the association of Zac1 with importin alpha1 is also involved in regulating the transactivation activity of Zac1 on the p21WAF1/CIP1 gene and protein expression.
Figures



Similar articles
-
Modulation of the cyclin-dependent kinase inhibitor p21(WAF1/Cip1) gene by Zac1 through the antagonistic regulators p53 and histone deacetylase 1 in HeLa Cells.Mol Cancer Res. 2008 Jul;6(7):1204-14. doi: 10.1158/1541-7786.MCR-08-0123. Mol Cancer Res. 2008. PMID: 18644983
-
Functional domains of the TGF-beta-inducible transcription factor Tieg3 and detection of two putative nuclear localization signals within the zinc finger DNA-binding domain.J Cell Biochem. 2007 Jun 1;101(3):712-22. doi: 10.1002/jcb.21228. J Cell Biochem. 2007. PMID: 17252542
-
Zac1, an Sp1-like protein, regulates human p21(WAF1/Cip1) gene expression in HeLa cells.Exp Cell Res. 2011 Dec 10;317(20):2925-37. doi: 10.1016/j.yexcr.2011.09.018. Epub 2011 Oct 6. Exp Cell Res. 2011. PMID: 22001409
-
Interaction of Sp1 zinc finger with transport factor in the nuclear localization of transcription factor Sp1.Biochem Biophys Res Commun. 2010 Dec 10;403(2):161-6. doi: 10.1016/j.bbrc.2010.10.036. Epub 2010 Oct 12. Biochem Biophys Res Commun. 2010. PMID: 20946882
-
Role of ZAC1 in transient neonatal diabetes mellitus and glucose metabolism.World J Biol Chem. 2015 Aug 26;6(3):95-109. doi: 10.4331/wjbc.v6.i3.95. World J Biol Chem. 2015. PMID: 26322169 Free PMC article. Review.
Cited by
-
Plag1 and Plagl2 have overlapping and distinct functions in telencephalic development.Biol Open. 2018 Nov 26;7(11):bio038661. doi: 10.1242/bio.038661. Biol Open. 2018. PMID: 30361413 Free PMC article.
-
Functional and structural basis of the nuclear localization signal in the ZIC3 zinc finger domain.Hum Mol Genet. 2008 Nov 15;17(22):3459-73. doi: 10.1093/hmg/ddn239. Epub 2008 Aug 20. Hum Mol Genet. 2008. PMID: 18716025 Free PMC article.
-
Zac1 regulates IL-11 expression in osteoarthritis.Oncotarget. 2018 Aug 21;9(65):32478-32495. doi: 10.18632/oncotarget.25980. eCollection 2018 Aug 21. Oncotarget. 2018. PMID: 30197757 Free PMC article.
-
PLAGL1 gene function during hepatoma cells proliferation.Oncotarget. 2018 Aug 28;9(67):32775-32794. doi: 10.18632/oncotarget.25996. eCollection 2018 Aug 28. Oncotarget. 2018. PMID: 30214684 Free PMC article.
-
Different Isoforms of HPV-16 E7 Protein are Present in Cytoplasm and Nucleus.Open Virol J. 2008;2:15-23. doi: 10.2174/1874357900802010015. Epub 2008 Mar 26. Open Virol J. 2008. PMID: 19440460 Free PMC article.
References
-
- Varrault A., Ciani E., Apiou F., Bilanges B., Hoffmann A., Pantaloni C., Bockaert J., Spengler D., Journot L. hZAC encodes a zinc finger protein with antiproliferative properties and maps to a chromosomal region frequently lost in cancer. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8835–8840. - PMC - PubMed
-
- Kas K., Voz M. L., Roijer E., Astrom A. K., Meyen E., Stenman G., Van de Ven W. J. Promoter swapping between the genes for a novel zinc finger protein and β-catenin in pleiomorphic adenomas with t(3;8)(p21;q12) translocations. Nat. Genet. 1997;15:170–174. - PubMed
-
- Kas K., Voz M. L., Hensen K., Meyen E., Van de Ven W. J. Transcriptional activation capacity of the novel PLAG family of zinc finger proteins. J. Biol. Chem. 1998;273:23026–23032. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

