Data mining of NCI's anticancer screening database reveals mitochondrial complex I inhibitors cytotoxic to leukemia cell lines
- PMID: 17109823
- PMCID: PMC1808352
- DOI: 10.1016/j.bcp.2006.10.005
Data mining of NCI's anticancer screening database reveals mitochondrial complex I inhibitors cytotoxic to leukemia cell lines
Abstract
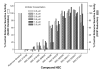
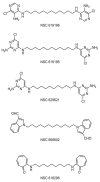
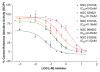
Mitochondria are principal mediators of apoptosis and thus can be considered molecular targets for new chemotherapeutic agents in the treatment of cancer. Inhibitors of mitochondrial complex I of the electron transport chain have been shown to induce apoptosis and exhibit antitumor activity. In an effort to find novel complex I inhibitors which exhibited anticancer activity in the NCI's tumor cell line screen, we examined organized tumor cytotoxicity screening data available as SOM (self-organized maps) (http://www.spheroid.ncifcrf.gov) at the developmental therapeutics program (DTP) of the National Cancer Institute (NCI). Our analysis focused on an SOM cluster comprised of compounds which included a number of known mitochondrial complex I (NADH:CoQ oxidoreductase) inhibitors. From these clusters 10 compounds whose mechanism of action was unknown were tested for inhibition of complex I activity in bovine heart sub-mitochondrial particles (SMP) resulting in the discovery that 5 of the 10 compounds demonstrated significant inhibition with IC50's in the nM range for three of the five. Examination of screening profiles of the five inhibitors toward the NCI's tumor cell lines revealed that they were cytotoxic to the leukemia subpanel (particularly K562 cells). Oxygen consumption experiments with permeabilized K562 cells revealed that the five most active compounds inhibited complex I activity in these cells in the same rank order and similar potency as determined with bovine heart SMP. Our findings thus fortify the appeal of mitochondrial complex I as a possible anticancer molecular target and provide a data mining strategy for selecting candidate inhibitors for further testing.
Figures
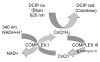
References
-
- Ferreira CG, Epping M, Kruyt FAE, Giaccone G. Apoptosis: Target of cancer therapy. Clin Cancer Res. 2002;8:2024–2034. - PubMed
-
- Makin G. Targeting apoptosis in cancer chemotherapy. Expert Opin Ther Targets. 2002;6:73–84. - PubMed
-
- Kaufman SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256:42–49. - PubMed
-
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. - PubMed
-
- Yano T. The energy-transducing NADH: quinone oxidoreductase, complex I. Mol Aspects Med. 2002;23:345–368. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

