H2AX chromatin structures and their response to DNA damage revealed by 4Pi microscopy
- PMID: 17110439
- PMCID: PMC1636994
- DOI: 10.1073/pnas.0608709103
H2AX chromatin structures and their response to DNA damage revealed by 4Pi microscopy
Abstract
DNA double-strand breaks (DSBs) caused by cellular exposure to genotoxic agents or produced by inherent metabolic processes initiate a rapid and highly coordinated series of molecular events resulting in DNA damage signaling and repair. Phosphorylation of histone H2AX to form gamma-H2AX is one of the earliest of these events and is important for coordination of signaling and repair activities. An intriguing aspect of H2AX phosphorylation is that gamma-H2AX spreads a limited distance up to 1-2 Mbp from the site of a DNA break in mammalian cells. However, neither the distribution of H2AX throughout the genome nor the mechanism that defines the boundary of gamma-H2AX spreading have yet been described. Here, we report the identification of previously undescribed H2AX chromatin structures by successfully applying 4Pi microscopy to visualize endogenous nuclear proteins. Our observations suggest that H2AX is not distributed randomly throughout bulk chromatin, rather it exists in distinct clusters that themselves are uniformly distributed within the nuclear volume. These data support a model in which the size and distribution of H2AX clusters define the boundaries of gamma-H2AX spreading and also may provide a platform for the immediate and robust response observed after DNA damage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

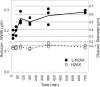
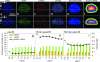


References
-
- O'Driscoll M, Jeggo PA. Nat Rev Genet. 2006;7:45–54. - PubMed
-
- Stucki M, Jackson SP. DNA Repair. 2006;5:534–543. - PubMed
-
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. J Biol Chem. 1998;273:5858–5868. - PubMed
-
- Thiriet C, Hayes JJ. Mol Cell. 2005;18:617–622. - PubMed
-
- Peterson CL, Cote J. Genes Dev. 2004;18:602–616. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

