Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex
- PMID: 17110440
- PMCID: PMC1636993
- DOI: 10.1073/pnas.0608545103
Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex
Abstract
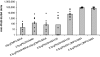
The development of protein subunit vaccines to combat some of the world's deadliest pathogens such as a malaria parasite, Plasmodium falciparum, is stalled, due in part to the inability to induce and sustain high-titer antibody responses. Here, we show the induction of persistent, high-titer antibody responses to recombinant Pfs25H, a human malarial transmission-blocking protein vaccine candidate, after chemical conjugation to the outer-membrane protein complex (OMPC) of Neisseria meningitidis serogroup B and adsorption to aluminum hydroxyphosphate. In mice, the Pfs25H-OMPC conjugate vaccine was >1,000 times more potent in generating anti-Pfs25H ELISA reactivity than a similar 0.5-microg dose of Pfs25H alone in Montanide ISA720, a water-in-oil adjuvant. The immune enhancement requires covalent conjugation between Pfs25H and the OMPC, given that physically mixed Pfs25H and OMPC on aluminum hydroxyphosphate failed to induce greater activity than the nonconjugated Pfs25H on aluminum hydroxyphosphate. The conjugate vaccine Pfs25H-OMPC also was highly immunogenic in rabbits and rhesus monkeys. In rhesus monkeys, the antibody responses were sustained over 18 months, at which time another vaccination with nonconjugated Pfs25H induced strong anamnestic responses. The vaccine-induced anti-Pfs25-specific antibodies in all animal species blocked the transmission of parasites to mosquitoes. Protein antigen conjugation to OMPC or other protein carrier may have general application to a spectrum of protein subunit vaccines to increase immunogenicity without the need for potentially reactogenic adjuvants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

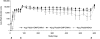
References
-
- World Health Organization. Malaria: Disease Information. Geneva: WHO; 2004. available at www.who.int/tdr/diseases/malaria/diseaseinfo.html, accessed.
-
- Carter R, Mendis KN, Miller LH, Molineaux L, Saul A. Nat Med. 2000;6:241–244. - PubMed
-
- Donnelly JJ, Deck RR, Liu MA. J Immunol. 1990;145:3071–3079. - PubMed
-
- Vella PP, Staub JM, Armstrong J, Dolan KT, Rusk CM, Szymanski S, Greer WE, Marburg S, Kniskern PJ, Schofield TL, et al. Pediatrics. 1990;85:668–675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

