Regulation of protein synthesis by the heme-regulated eIF2alpha kinase: relevance to anemias
- PMID: 17110456
- PMCID: PMC1852217
- DOI: 10.1182/blood-2006-08-041830
Regulation of protein synthesis by the heme-regulated eIF2alpha kinase: relevance to anemias
Abstract
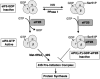
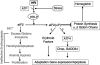
During erythroid differentiation and maturation, it is critical that the 3 components of hemoglobin, alpha-globin, beta-globin, and heme, are made in proper stoichiometry to form stable hemoglobin. Heme-regulated translation mediated by the heme-regulated inhibitor kinase (HRI) provides one major mechanism that ensures balanced synthesis of globins and heme. HRI phosphorylates the alpha-subunit of eukaryotic translational initiation factor 2 (eLF2alpha) in heme deficiency, thereby inhibiting protein synthesis globally. In this manner, HRI serves as a feedback inhibitor of globin synthesis by sensing the intracellular concentration of heme through its heme-binding domains. HRI is essential not only for the translational regulation of globins, but also for the survival of erythroid precursors in iron deficiency. Recently, the protective function of HRI has also been demonstrated in murine models of erythropoietic protoporphyria and beta-thalassemia. In these 3 anemias, HRI is essential in determining red blood cell size, number, and hemoglobin content per cell. Translational regulation by HRI is critical to reduce excess synthesis of globin proteins or heme under nonoptimal disease states, and thus reduces the severity of these diseases. The protective role of HRI may be more common among red cell disorders.
Figures


References
-
- Ponka P. Tissue-specific regulation of iron metabolism and heme synthesis: distinct control mechanisms in erythroid cells. Blood. 1997;89:1–25. - PubMed
-
- Taketani S. Aquisition, mobilization and utilization of cellular iron and heme: endless findings and growing evidence of tight regulation. Tohoku J Exp Med. 2005;205:297–318. - PubMed
-
- Igarashi K, Sun J. The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxid Redox Signal. 2006;8:107–118. - PubMed
-
- Chen J-J. Heme-regulated eIF-2α kinase. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 529–546.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

