Structural mechanism of RPA loading on DNA during activation of a simple pre-replication complex
- PMID: 17110927
- PMCID: PMC1679769
- DOI: 10.1038/sj.emboj.7601432
Structural mechanism of RPA loading on DNA during activation of a simple pre-replication complex
Abstract
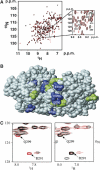
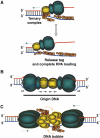
We report that during activation of the simian virus 40 (SV40) pre-replication complex, SV40 T antigen (Tag) helicase actively loads replication protein A (RPA) on emerging single-stranded DNA (ssDNA). This novel loading process requires physical interaction of Tag origin DNA-binding domain (OBD) with the RPA high-affinity ssDNA-binding domains (RPA70AB). Heteronuclear NMR chemical shift mapping revealed that Tag-OBD binds to RPA70AB at a site distal from the ssDNA-binding sites and that RPA70AB, Tag-OBD, and an 8-nucleotide ssDNA form a stable ternary complex. Intact RPA and Tag also interact stably in the presence of an 8-mer, but Tag dissociates from the complex when RPA binds to longer oligonucleotides. Together, our results imply that an allosteric change in RPA quaternary structure completes the loading reaction. A mechanistic model is proposed in which the ternary complex is a key intermediate that directly couples origin DNA unwinding to RPA loading on emerging ssDNA.
Figures




References
-
- Alexandrov AI, Botchan MR, Cozzarelli NR (2002) Characterization of Simian Virus 40 T-antigen double hexamers bound to a replication fork. The active form of helicase. J Biol Chem 277: 44886–44897 - PubMed
-
- Arunkumar AI, Stauffer ME, Bochkareva E, Bochkarev A, Chazin WJ (2003) Independent and coordinated functions of replication protein A tandem high affinity single-stranded DNA binding domains. J Biol Chem 278: 41077–41082 - PubMed
-
- Bhattacharya S, Arunkumar AI, Sullivan SL, Botuyan M-V, Arrowsmith CH, Chazin WJ (2004) Letter to the editor: 1H, 13C and 15N assignments of single-stranded DNA binding domains from the 70 kDa subunit of human replication protein A. J Biomol NMR 28: 195–196 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

