Comparison of p53 mutations induced by PAH o-quinones with those caused by anti-benzo[a]pyrene diol epoxide in vitro: role of reactive oxygen and biological selection
- PMID: 17112231
- PMCID: PMC2366885
- DOI: 10.1021/tx0601206
Comparison of p53 mutations induced by PAH o-quinones with those caused by anti-benzo[a]pyrene diol epoxide in vitro: role of reactive oxygen and biological selection
Abstract
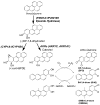
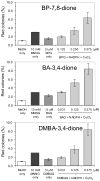
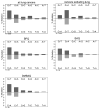
Polycyclic aromatic hydrocarbons (PAH) are one of the major carcinogens in tobacco smoke. They are metabolically activated through different routes to form either diol-epoxides, PAH o-quinones, or radical cations, each of which has been proposed to be an ultimate carcinogen. To study how PAH metabolites mutate p53, we used a yeast reporter gene assay based on p53 transcriptional activity. Colonies expressing wt p53 turn white (ADE +) and those expressing mutant p53 turn red (ADE -). We examined the mutagenicity of three o-quinones, benzo[a]pyrene-7,8-dione, benz[a]anthracene-3,4-dione, and dimethylbenz[a]anthracene-3,4-dione, and compared them with (+/-)-anti-benzo[a]pyrene diol epoxide ((+/-)-anti-BPDE) within the same system. The PAH o-quinones tested gave a dose-dependent increase in mutation frequency in the range of 0.160-0.375 microM quinone, provided redox-cycling conditions were used. The dominant mutations were G to T transversions (>42%), and the incidence of hotspot mutations in the DNA-binding domain was more than twice than that expected by a random distribution. The dependence of G to T transversions on redox cycling implicates 8-oxo-dGuo as the lesion responsible, which is produced under identical conditions (Chem. Res. Toxicol. (2005) 18, 1027). A dose-dependent mutation frequency was also observed with (+/-)-anti-BPDE but at micromolar concentrations (0-20 microM). The mutation pattern observed was G to C (63%) > G to A (18%) > G to T (15%) in umethylated p53 and was G to A (39%) > G to C (34%) > G to T (16%) in methylated p53. The preponderance of G mutations is consistent with the formation of anti-BPDE-N2-dGuo as the major adduct. The frequency of hotspots mutated by (+/-)-anti-BPDE was essentially random in umethylated and methylated p53, suggesting that 5'-CpG-3' islands did not direct mutations in the assay. These data suggest that smoking may cause mutations in p53 by formation of PAH o-quinones, which produce reactive oxygen species. The resultant 8-oxo-dGuo yields a pattern of mutations but not a spectrum consistent with that seen in lung cancer; we suggest that the emergence of the spectrum requires biological selection.
Figures




Similar articles
-
Aldo-keto reductases protect lung adenocarcinoma cells from the acute toxicity of B[a]P-7,8-trans-dihydrodiol.Chem Res Toxicol. 2012 Jan 13;25(1):113-21. doi: 10.1021/tx200272v. Epub 2011 Nov 16. Chem Res Toxicol. 2012. PMID: 22053912 Free PMC article.
-
Reactive oxygen species generated by PAH o-quinones cause change-in-function mutations in p53.Chem Res Toxicol. 2002 Jun;15(6):832-42. doi: 10.1021/tx010177m. Chem Res Toxicol. 2002. PMID: 12067251
-
The pattern of p53 mutations caused by PAH o-quinones is driven by 8-oxo-dGuo formation while the spectrum of mutations is determined by biological selection for dominance.Chem Res Toxicol. 2008 May;21(5):1039-49. doi: 10.1021/tx700404a. Chem Res Toxicol. 2008. PMID: 18489080 Free PMC article.
-
The molecular etiology and prevention of estrogen-initiated cancers: Ockham's Razor: Pluralitas non est ponenda sine necessitate. Plurality should not be posited without necessity.Mol Aspects Med. 2014 Apr;36:1-55. doi: 10.1016/j.mam.2013.08.002. Epub 2013 Aug 30. Mol Aspects Med. 2014. PMID: 23994691 Free PMC article. Review.
-
Dihydrodiol dehydrogenase and its role in polycyclic aromatic hydrocarbon metabolism.Chem Biol Interact. 1993 Oct;89(1):1-34. doi: 10.1016/0009-2797(93)03203-7. Chem Biol Interact. 1993. PMID: 8221964 Review.
Cited by
-
Detoxication of benzo[a]pyrene-7,8-dione by sulfotransferases (SULTs) in human lung cells.J Biol Chem. 2012 Aug 24;287(35):29909-20. doi: 10.1074/jbc.M112.386052. Epub 2012 Jul 9. J Biol Chem. 2012. PMID: 22782890 Free PMC article.
-
Biological effects of carbon black nanoparticles are changed by surface coating with polycyclic aromatic hydrocarbons.Part Fibre Toxicol. 2017 Mar 21;14(1):8. doi: 10.1186/s12989-017-0189-1. Part Fibre Toxicol. 2017. PMID: 28327162 Free PMC article.
-
Detoxication of structurally diverse polycyclic aromatic hydrocarbon (PAH) o-quinones by human recombinant catechol-O-methyltransferase (COMT) via O-methylation of PAH catechols.J Biol Chem. 2011 Jul 22;286(29):25644-54. doi: 10.1074/jbc.M111.240739. Epub 2011 May 27. J Biol Chem. 2011. PMID: 21622560 Free PMC article.
-
Aldo-keto reductases protect lung adenocarcinoma cells from the acute toxicity of B[a]P-7,8-trans-dihydrodiol.Chem Res Toxicol. 2012 Jan 13;25(1):113-21. doi: 10.1021/tx200272v. Epub 2011 Nov 16. Chem Res Toxicol. 2012. PMID: 22053912 Free PMC article.
-
Lung-Enriched Mutations in the p53 Tumor Suppressor: A Paradigm for Tissue-Specific Gain of Oncogenic Function.Mol Cancer Res. 2019 Jan;17(1):3-9. doi: 10.1158/1541-7786.MCR-18-0357. Epub 2018 Sep 17. Mol Cancer Res. 2019. PMID: 30224539 Free PMC article. Review.
References
-
- Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, Schrag D, Jamison PA, Jemal A, Wu XC, Friedman C, Harlan L, Warren J, Anderson RM, Pickle LW. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–1427. - PubMed
-
- Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons. G.H.A Clowes Memorial Lecture. Cancer Res. 1982;42:4875–4917. - PubMed
-
- Gelboin HV, T’so POP. Benzo[a]pyrene metabolism, activation and carcinogenesis: Role and regulation of mixed function oxidases and related enzymes. Physiol Rev. 1980;60:1007–1166. - PubMed
-
- Sutter TR, Tang YM, Hayes CL, Wo Y-Y, Jabs PW, Li X, Yin H, Cody CW, Greenlee WF. cDNA sequence of a human dioxin-inducible mRNA identifies a new gene subfamily of cytochrome P450 that maps to chromosome 2. J Biol Chem. 1994;269:13092–13099. - PubMed
-
- Shimada T, Hayes CL, Yamazaki H, Amin S, Hecht SS, Guengerich FP, Sutter TR. Activation of chemically diverse procarcinogens by human cytochrome P450 1B1. Cancer Res. 1996;56:2979–2984. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

