Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase
- PMID: 17112726
- PMCID: PMC1850625
- DOI: 10.1016/j.cub.2006.10.061
Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase
Abstract
Background: The linkage between duplicated chromosomes (sister chromatids) is established during S phase by the action of cohesin, a multisubunit complex conserved from yeast to humans. Most cohesin dissociates from chromosome arms when the cell enters mitotic prophase, leading to the formation of metaphase chromosomes with two cytologically discernible chromatids. This process is known as sister-chromatid resolution. Although two mitotic kinases have been implicated in this process, it remains unknown exactly how the cohesin-mediated linkage is destabilized at a mechanistic level.
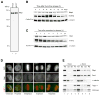
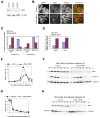
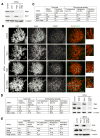
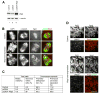
Results: The wings apart-like (Wapl) protein was originally identified as a gene product that potentially regulates heterochromatin organization in Drosophila melanogaster. We show that the human ortholog of Wapl is a cohesin-binding protein that facilitates cohesin's timely release from chromosome arms during prophase. Depletion of Wapl from HeLa cells causes transient accumulation of prometaphase-like cells with chromosomes that display poorly resolved sister chromatids with a high level of cohesin. Reduction of cohesin relieves the Wapl-depletion phenotype, and depletion of Wapl rescues premature sister separation observed in Sgo1-depleted or Esco2-depleted cells. Conversely, overexpression of Wapl causes premature separation of sister chromatids. Wapl physically associates with cohesin in HeLa-cell nuclear extracts. Remarkably, in vitro reconstitution experiments demonstrate that Wapl forms a stoichiometric, ternary complex with two regulatory subunits of cohesin, implicating its noncatalytic function in inactivating cohesin's ability to interact with chromatin.
Conclusions: Wapl is a new regulator of sister chromatid resolution and promotes release of cohesin from chromosomes by directly interacting with its regulatory subunits.
Figures


References
-
- Losada A, Hirano T. Dynamic molecular linkers of the genome: the first decade of SMC proteins. Genes Dev. 2005;19:1269–1287. - PubMed
-
- Nasmyth K, Haering CH. The structure and function of SMC and kleisin complexes. Annu Rev Biochem. 2005;74:595–648. - PubMed
-
- Hirano T. Chromosome cohesion, condensation and separation. Annu Rev Biochem. 2000;69:115–144. - PubMed
-
- Rajagopalan H, Lengauer C. Aneuploidy and cancer. Nature. 2004;432:338–341. - PubMed
-
- Draviam VM, Xie S, Sorger PK. Chromosome segregation and genomic stability. Curr Opin Genet Dev. 2004;14:120–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

