Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex
- PMID: 17113043
- PMCID: PMC1839952
- DOI: 10.1016/j.brainres.2006.09.110
Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex
Abstract
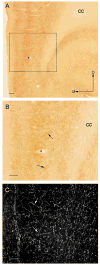
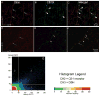
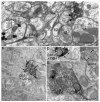
Cannabinoid agonists exert complex actions on modulatory neurotransmitters involved in attention and cognition. Previous studies have demonstrated that acute systemic administration of the synthetic cannabinoid agonist, WIN 55,212-2, increases norepinephrine efflux in the rat frontal cortex. In an effort to elucidate whether cannabinoid (CB1) receptors are positioned to presynaptically modulate norepinephrine release in the frontal cortex, immunocytochemical detection of the CB1 receptor and the catecholamine-synthesizing enzyme dopamine-beta-hydroxylase (DbetaH) was performed using confocal immunofluorescence microscopy and immunoelectron microscopy in rat brain. Fluorescence microscopy analysis of dually labeled tissue sections from the frontal cortex indicated that individual axonal processes exhibited both CB1 receptor and DbetaH immunoreactivities. Ultrastructural analysis confirmed that one-third of axon terminals containing CB1 immunolabeling also exhibited DbetaH labeling. Cortical neurons were also found to be targeted by separately labeled CB1- and DbetaH-containing axon terminals. In conclusion, the present neuroanatomical data suggest that cortical norepinephrine release may be modulated, in part, by CB1 receptors that are presynaptically distributed on noradrenergic axon terminals.
Figures
Similar articles
-
Ultrastructural evidence for synaptic contacts between cortical noradrenergic afferents and endocannabinoid-synthesizing post-synaptic neurons.Neuroscience. 2015 Sep 10;303:323-37. doi: 10.1016/j.neuroscience.2015.07.009. Epub 2015 Jul 8. Neuroscience. 2015. PMID: 26162236 Free PMC article.
-
Local administration of a cannabinoid agonist alters norepinephrine efflux in the rat frontal cortex.Neurosci Lett. 2008 Jan 24;431(1):1-5. doi: 10.1016/j.neulet.2007.11.009. Epub 2007 Nov 9. Neurosci Lett. 2008. PMID: 18055114 Free PMC article.
-
Systemic administration of WIN 55,212-2 increases norepinephrine release in the rat frontal cortex.Brain Res. 2005 Jun 7;1046(1-2):45-54. doi: 10.1016/j.brainres.2005.03.036. Brain Res. 2005. PMID: 15927549
-
Retrograde endocannabinoid signaling in the cerebellar cortex.Cerebellum. 2006;5(2):134-45. doi: 10.1080/14734220600791477. Cerebellum. 2006. PMID: 16818388 Review.
-
Modulatory effects of cannabinoids on brain neurotransmission.Eur J Neurosci. 2019 Aug;50(3):2322-2345. doi: 10.1111/ejn.14407. Epub 2019 Apr 8. Eur J Neurosci. 2019. PMID: 30882962 Review.
Cited by
-
Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice.PLoS Biol. 2007 Oct;5(10):e269. doi: 10.1371/journal.pbio.0050269. PLoS Biol. 2007. PMID: 17927447 Free PMC article.
-
Supraspinal modulation of pain by cannabinoids: the role of GABA and glutamate.Br J Pharmacol. 2007 Nov;152(5):633-48. doi: 10.1038/sj.bjp.0707440. Epub 2007 Sep 10. Br J Pharmacol. 2007. PMID: 17828292 Free PMC article. Review.
-
The role of cannabinoids in modulating emotional and non-emotional memory processes in the hippocampus.Front Behav Neurosci. 2011 Jun 23;5:34. doi: 10.3389/fnbeh.2011.00034. eCollection 2011. Front Behav Neurosci. 2011. PMID: 21734875 Free PMC article.
-
Potentiation of electrical and chemical synaptic transmission mediated by endocannabinoids.Neuron. 2007 Dec 20;56(6):1034-47. doi: 10.1016/j.neuron.2007.11.014. Neuron. 2007. PMID: 18093525 Free PMC article.
-
Abnormal cerebellar morphometry in abstinent adolescent marijuana users.Psychiatry Res. 2010 May 30;182(2):152-9. doi: 10.1016/j.pscychresns.2009.12.004. Epub 2010 Apr 21. Psychiatry Res. 2010. PMID: 20413277 Free PMC article.
References
-
- Acquas E, Pisanu A, et al. Cannabinoid CB(1) receptor agonists increase rat cortical and hippocampal acetylcholine release in vivo. Eur J Pharmacol. 2000;401(2):79–85. - PubMed
-
- Arnold JC, Topple AN, et al. The distribution of cannabinoid-induced Fos expression in rat brain: differences between the Lewis and Wistar strain. Brain Res. 2001;921(1–2):240–55. - PubMed
-
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11(2):151–62. - PubMed
-
- Aston-Jones G. Behavioral functions of locus coeruleus derived from cellular attributes. Physiol Psychol. 1985;13:118–126.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

