Endothelial nitric oxide synthase keeps erection regulatory function balance in the penis
- PMID: 17113219
- PMCID: PMC3328507
- DOI: 10.1016/j.eururo.2006.10.061
Endothelial nitric oxide synthase keeps erection regulatory function balance in the penis
Abstract
Objectives: We evaluated the regulatory influence of endothelial nitric oxide (NO) on the basal functional states of the NO and RhoA/Rho-kinase signaling pathways in the penis using endothelial NO synthase (eNOS) mutant mice and eNOS gene transfer technology.
Methods: Four groups of mice were used: wild type (WT), eNOS gene deleted (eNOS-/-), eNOS and neuronal NOS gene deleted (dNOS-/-), and eNOS-/- mutant mice transfected intracavernosally with eNOS. Cyclic guanosine monophosphate (cGMP) concentration, protein kinase G (PKG) activity, activated RhoA, and Rho-kinase activity were determined in penes of WT and both mutant mouse groups. Constitutive NOS and PKG activities, RhoA, Rho-kinase-alpha and -beta isoforms, and phosphorylated myosin light-chain phosphatase target subunit (p-MYPT-1) expressions and Rho-kinase activity were determined in penes of eNOS-/- mice after eNOS gene transfer.
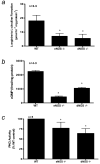
Results: Compared with results in the WT penis, eNOS-/- and dNOS-/- mutant mouse penes had significant reductions in NOS activity, cGMP concentration, PKG activity, Rho-kinase activity, and p-MYPT-1 expression (p<0.05) with no significant changes in activated RhoA or in RhoA and Rho-kinase-alpha and -beta protein expressions. After eNOS gene transfer to penes of eNOS-/- mice, Rho-kinase-beta and p-MYPT-1 expressions and total Rho-kinase activity were significantly increased from baseline levels (p<0.05).
Conclusions: These data suggest that endothelial NO has a role in the penis as a regulator of the basal signaling functions of the NO and RhoA/Rho-kinase erection mediatory pathways. These data offer new insight into the homeostasis of erection regulatory biology.
Figures




References
-
- Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992;326:90–4. - PubMed
-
- Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257:401–3. - PubMed
-
- Bivalacqua TJ, Usta MF, Champion HC, Kadowitz PJ, Hellstrom WJG. Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J Androl. 2003;24:S17–37. - PubMed
-
- Chitaley K, Wingard CJ, Webb CR, et al. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med. 2001;7:119–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

