Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists
- PMID: 17113298
- PMCID: PMC1963459
- DOI: 10.1016/j.bmc.2006.10.060
Synthesis and biological evaluation of gamma-aminophosphonates as potent, subtype-selective sphingosine 1-phosphate receptor agonists and antagonists
Abstract
The synthesis of N-arylamide phosphonates and related arylether and arylamine analogues provided potent, subtype-selective agonists and antagonists of the five known sphingosine 1-phosphate (S1P) receptors (S1P(1-5)). To this end, the syntheses of phosphoserine mimetics-selectively protected and optically active phosphonoserines-are described. In vitro binding assays showed that the implementation of phosphonates as phosphate mimetics provided compounds with similar receptor binding affinities as compared to their phosphate precursors. meta-substituted arylamide phosphonates were discovered to be antagonists of the S1P(1) and S1P(3) receptors. When administered to mice, an antagonist blocked the lymphopenia evoked by a S1P receptor agonist and caused capillary leakage in both lung and kidney.
Figures

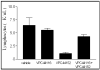
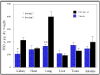
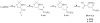

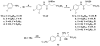
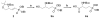

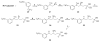
References
-
- Lynch KR. Biochim et Biophys Acta. 2002;1582:70–71. - PubMed
-
- Hla T. Pharmacol Rev. 2003;47:401–407. - PubMed
-
- Watterson K, Sankala H, Milstien S, Spiegel S. Prog in Lipid Res. 2003;42:344–357. - PubMed
-
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Nature. 2004;427:355–360. - PubMed
-
-
For a convenient review: Im D-S. TRENDS in Pharmacol Sci. 2003;24:12–4.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

