A member of the cathelicidin family of antimicrobial peptides is produced in the upper airway of the chinchilla and its mRNA expression is altered by common viral and bacterial co-pathogens of otitis media
- PMID: 17113647
- PMCID: PMC1817667
- DOI: 10.1016/j.molimm.2006.10.008
A member of the cathelicidin family of antimicrobial peptides is produced in the upper airway of the chinchilla and its mRNA expression is altered by common viral and bacterial co-pathogens of otitis media
Abstract
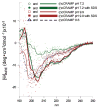
Cationic antimicrobial peptides (AMPs), a component of the innate immune system, play a major role in defense of mucosal surfaces against a wide spectrum of microorganisms such as viral and bacterial co-pathogens of the polymicrobial disease otitis media (OM). To further understand the role of AMPs in OM, we cloned a cDNA encoding a cathelicidin homolog (cCRAMP) from upper respiratory tract (URT) mucosae of the chinchilla, the predominant host used to model experimental OM. Recombinant cCRAMP exhibited alpha-helical secondary structure and killed the three main bacterial pathogens of OM. In situ hybridization showed cCRAMP mRNA production in epithelium of the chinchilla Eustachian tube and RT-PCR was used to amplify cCRAMP mRNA from several other tissues of the chinchilla URT. Quantitative RT-PCR analysis of chinchilla middle ear epithelial cells (CMEEs) incubated with either viral (influenza A virus, adenovirus, or RSV) or bacterial (nontypeable H. influenzae, M. catarrhalis, or S. pneumoniae) pathogens associated with OM demonstrated distinct microbe-specific patterns of altered expression. Collectively, these data showed that viruses and bacteria modulate AMP messages in the URT, which likely contributes to the disease course of OM.
Figures





References
-
- Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jornvall H, Wigzell H, Gudmundsson GH. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. - PubMed
-
- Alsarraf R, Jung CJ, Perkins J, Crowley C, Alsarraf NW, Gates GA. Measuring the indirect and direct costs of acute otitis media. Arch Otolaryngol Head Neck Surg. 1999;125:12–18. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. John Wiley & Sons, Inc; 1993.
-
- Bakaletz LO. Otitis Media. In: Brogden KA, Guthmiller JM, editors. Polymicrobial Diseases. ASM Press; Washington, D.C: 2002. pp. 259–298. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

