Aromatase and breast cancer
- PMID: 17113978
- PMCID: PMC1868399
- DOI: 10.1016/j.jsbmb.2006.09.002
Aromatase and breast cancer
Abstract
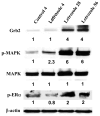
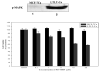
Several aromatase inhibitors and also new antiestrogens are now available for treating breast cancer. We have developed a model to compare the antitumor efficacy of these agents and to explore strategies for their optimal use. Results from the model have been predictive of clinical outcome. In this model, tumors are grown in ovariectomized, immunodeficient mice from MCF-7 human breast cancer cells transfected with the aromatase gene (MCF-7Ca). The possibility that blockade of estrogen action and estrogen synthesis may be synergistic was explored by treating mice with the aromatase inhibitor letrozole and the antiestrogen tamoxifen alone and in combination. The results indicated that letrozole alone was better than all other treatments. In addition, when tamoxifen treatment was no longer effective, tumor growth was significantly reduced in mice switched to letrozole treatment. However, tumors ultimately began to grow during continued treatment. To investigate the mechanisms by which tumors eventually adapt and grow during letrozole treatment, we determined the expression of signaling proteins in tumors during the course of letrozole treatment compared to the tumors of control mice. Tumors initially up-regulated the ER while responding to treatment, but subsequently receptor levels decreased in tumors unresponsive to letrozole. Also, Her-2 and adapter proteins (p-Shc and Grb-2) as well as all of the signaling proteins in the MAPK cascade (p-Raf, p-Mekl/2, and p-MAPK), but not in the Pl3/Akt pathway, were increased in tumors no longer responsive to letrozole. To investigate whether sensitivity to letrozole could be regained, cells were isolated from the letrozole resistant tumors (LTLT) and treated with inhibitors of the MAPKinase pathway (PD98059 and UO126). These compounds reduced MAPK activity and increased ER expression. EGFR/Her-2 inhibitors, gefitinib and AEE78S although not effective in the parental MCF-70a cells, restored the sensitivity of LTLT cells to letrozole. In xenografts, beginning treatment with letrozole and faslodex to down regulate the ER prevented increases in Her-2 and activation of MAPK and was highly effective in inhibiting tumor growth throughout 29 weeks of treatment. These results suggest that blocking both ER- and growth factor-mediated transcription may delay development of resistance and maintain growth inhibition of ER+ breast cancer.
Figures

References
-
- Yue W, Zhou DJ, Chen S, Brodie AMH. A new nude mouse model for postmenopausal breast cancer using MCF-7 cells transfected with the human aromatase gene. Cancer Res. 1994;54:5092–5095. - PubMed
-
- Yue W, Wang J, Savinov A, Brodie A. Effect of aromatase inhibitors on the growth of mammary tumors in a nude mouse model. Cancer Res. 1995;55:3073–3077. - PubMed
-
- Zhou D, Pompon D, Chen S. Stable expression of human aromatase complementary DNA in mammalian cells: a useful system for aromatase inhibitor screening. Cancer Res. 1990;50:6949–6954. - PubMed
-
- Lu Q, Wang J, Liu Y, Long B, Brodie A. The effects of aromatase and antiestrogens in the nude mouse model. Breast Cancer Res Treat. 1998;50:63–71. - PubMed
-
- Lu Q, Liu Y, Long BJ, Grigoryev D, Gimbel M, Brodie A. The effect of combining aromatase inhibitors with antiestrogens on tumor growth in a nude mouse model for breast cancer. Breast Cancer Res Treat. 1999;57:183–192. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

