FRET study of membrane proteins: determination of the tilt and orientation of the N-terminal domain of M13 major coat protein
- PMID: 17114224
- PMCID: PMC1783881
- DOI: 10.1529/biophysj.106.095026
FRET study of membrane proteins: determination of the tilt and orientation of the N-terminal domain of M13 major coat protein
Abstract
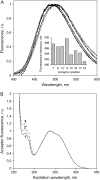
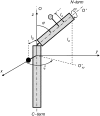
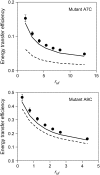
A formalism for membrane protein structure determination was developed. This method is based on steady-state FRET data and information about the position of the fluorescence maxima on site-directed fluorescent labeled proteins in combination with global data analysis utilizing simulation-based fitting. The methodology was applied to determine the structural properties of the N-terminal domain of the major coat protein from bacteriophage M13 reconstituted into unilamellar DOPC/DOPG (4:1 mol/mol) vesicles. For our purpose, the cysteine mutants A7C, A9C, N12C, S13C, Q15C, A16C, S17C, and A18C in the N-terminal domain of this protein were produced and specifically labeled with the fluorescence probe AEDANS. The energy transfer data from the natural Trp-26 to AEDANS were analyzed assuming a two-helix protein model. Furthermore, the polarity Stokes shift of the AEDANS fluorescence maxima is taken into account. As a result the orientation and tilt of the N-terminal protein domain with respect to the bilayer interface were obtained, showing for the first time, to our knowledge, an overall alpha-helical protein conformation from amino acid residues 12-46, close to the protein conformation in the intact phage.
Figures


References
-
- Stopar, D., R. B. Spruijt, and M. A. Hemminga. 2006. Anchoring mechanisms of membrane-associated M13 major coat protein. Chem. Phys. Lipids. 141:83–93. - PubMed
-
- Vos, W. L., R. B. M. Koehorst, R. B. Spruijt, and M. A. Hemminga. 2005. Membrane-bound conformation of M13 major coat protein: a structure validations through FRET-derived constrains. J. Biol. Chem. 280:38522–38527. - PubMed
-
- Spruijt, R. B., A. B. Meijer, C. J. A. M. Wolfs, and M. A. Hemminga. 2000. Localization and rearrangement modulation of the N-terminal arm of the membrane-bound major coat protein of bacteriophage M13. Biochim. Biophys. Acta. 1509:311–323. - PubMed
-
- Spruijt, R. B., C. J. A. M. Wolfs, and M. A. Hemminga. 1989. Aggregation-related conformational change of the membrane-associated coat protein of bacteriophage M13. Biochemistry. 28:9158–9165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

