Tobacco nectaries express a novel NADPH oxidase implicated in the defense of floral reproductive tissues against microorganisms
- PMID: 17114277
- PMCID: PMC1761964
- DOI: 10.1104/pp.106.089326
Tobacco nectaries express a novel NADPH oxidase implicated in the defense of floral reproductive tissues against microorganisms
Abstract
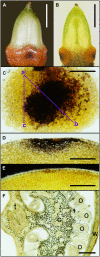
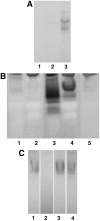
Hydrogen peroxide produced from the nectar redox cycle was shown to be a major factor contributing to inhibition of most microbial growth in floral nectar; however, this obstacle can be overcome by the floral pathogen Erwinia amylovora. To identify the source of superoxide that leads to hydrogen peroxide accumulation in nectary tissues, nectaries were stained with nitroblue tetrazolium. Superoxide production was localized near nectary pores and inhibited by diphenylene iodonium but not by cyanide or azide, suggesting that NAD(P)H oxidase is the source of superoxide. Native PAGE assays demonstrated that NADPH (not NADH) was capable of driving the production of superoxide, diphenyleneiodonium chloride was an efficient inhibitor of this activity, but cyanide and azide did not inhibit. These results confirm that the production of superoxide was due to an NADPH oxidase. The nectary enzyme complex was distinct by migration on gels from the leaf enzyme complex. Temporal expression patterns demonstrated that the superoxide production (NADPH oxidase activity) was coordinated with nectar secretion, the expression of Nectarin I (a superoxide dismutase in nectar), and the expression of NOX1, a putative gene for a nectary NADPH oxidase that was cloned from nectaries and identified as an rbohD-like NADPH oxidase. Further, in situ hybridization studies indicated that the NADPH oxidase was expressed in the early stages of flower development although superoxide was generated at later stages (after Stage 10), implicating posttranslational regulation of the NADPH oxidase in the nectary.
Figures






References
-
- Ankri S, Mirelman D (1999) Antimicrobial properties of allicin from garlic. Microbes Infect 1 125–129 - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1992) Short Protocols in Molecular Biology, Ed 2. John Wiley & Sons, New York
-
- Baker HG, Baker I (1971) Amino acids in nectar and their evolutionary significance. Nature 241 543–545
-
- Bokoch GM, Diebold BA (2002) Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood 100 2692–2696 - PubMed
-
- Bolwell G, Butt V, Davies D, Zimmerlin A (1995) The origin of the oxidative burst in plants. Free Radic Res 23 517–532 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

